| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://cmmr.elmerpub.com |
Original Article
Volume 000, Number 000, July 2025, pages 000-000
Screening for Light Chain Monoclonal Gammopathy of Undetermined Significance: Pseudo Epidemic Based on Current Diagnostic Criteria
Gurmukh Singha, c, Hongyan Xub, Roni J. Bollaga
aDepartment of Pathology, Medical College of Georgia at Augusta University, Augusta, GA 30912, USA
bDepartment of Biostatistics, Data Science and Epidemiology, School of Public Health at Augusta University, Augusta, GA 30912, USA
cCorresponding Author: Department of Pathology, Medical College of Georgia at Augusta University, Augusta, GA 30912, USA
Manuscript submitted February 11, 2025, accepted April 10, 2025, published online July 7, 2025
Short title: Light Chain Monoclonal Gammopathy
doi: https://doi.org/10.14740/cmmr103
| Abstract | ▴Top |
Background: An abnormal serum free light chain ratio has been promoted as a marker for light chain monoclonal gammopathy, even in the absence of any other evidence of lympho-plasmacytic pathology. The diagnostic ratio has been used primarily to support the diagnosis of kappa light chain monoclonal gammopathy of undetermined significance (MGUS). The recommended diagnostic ratio has varied from > 1.65 to > 3.15.
Methods: Serum free light chain values as well as serum and urine immunofixation results were retrieved from medical records for the period of January 1, 2010, to June 30, 2024. Laboratory and clinical data for 4,998 specimens were reviewed to ascertain the presence/absence of monoclonal gammopathy.
Results: In patients with no other evidence of lympho-plasmacytic monoclonal disorder: 1) three of seven specimens with κ/λ ratio < 0.25 exhibited monoclonal lambda light chains in urine; 2) in specimens with κ/λ ratio of 1.66 to 2.9, 13 of 547 (2.4%) had monoclonal kappa light chains in urine; 3) in specimens with κ/λ ratio of 3 - 353, ten of 53 (18.8%) demonstrated monoclonal kappa light chains in urine.
Conclusions: Diagnosis of patients with a κ/λ ratio of > 3 or 3.15 as having kappa chain MGUS would result in an unsupported diagnosis, pseudo epidemic, of a premalignant lesion in > 80% of instances. The serum free light chain ratio is not a reliable parameter for diagnosing or excluding light chain monoclonal gammopathy. Before a patient is given the diagnosis of a pre-malignant monoclonal disorder, monoclonality of immunoglobulins ought to be documented, or there becomes a risk of generating a “pseudo epidemic” of MGUS diagnoses.
Keywords: Serum free light chains; Light chain monoclonal gammopathy; Light chain predominant multiple myeloma; Monoclonal gammopathy of undetermined significance; FLC-UIFE
| Introduction | ▴Top |
Monoclonal gammopathies (MGs) comprise clonal proliferations of terminally differentiated B lymphocytes and monoclonal immunoglobulin production [1, 2]. Neoplastic monoclonal gammopathies (NMGs) include monoclonal gammopathy of undetermined significance (MGUS), smoldering/asymptomatic multiple myeloma (SMM), and multiple myeloma (MM) [3-6]. MGUS and SMM are pre-malignant disorders. MM is the second most common hematological malignancy in adults and accounts for about 2% of cancer deaths [7-10].
About 85% of MMs secrete intact monoclonal immunoglobulins and variable quantities of free monoclonal light chains (FMLCs) [6]. About 18% of these lesions produce excessive FMLCs and are called light chain predominant multiple myelomas (LCPMMs). Patients with LCPMMs have a 2-year shorter survival compared to patients with conventional MM [11-14]. About 15% of MMs secrete light chains only and are called light chain MMs (LCMMs) [6]. Thus, about one-third of MMs produce excess free monoclonal light chains [11-14].
Normal and neoplastic plasma cells empirically synthesize more light chains than heavy chains, leading to detection of free light chains (FLCs) in serum and urine [6, 15-21]. Bradwell identified epitopes on FLCs that are hidden in intact immunoglobulins [22]. Antisera specific to FLCs were used in quantification of FLCs in serum. Reference ranges for serum free light chains (SFLCs) were established, and a normal range for κ/λ light chain ratio was set at 0.25 to 1.65 [22-24]. The ratio is affected by NMG disorders, polyclonal proliferation of lympho-plasmacytic cells, and renal failure [6, 17-21, 25]. Documentation of an abnormal ratio of SFLC (κ/λ ratio) has been promoted as a laboratory test to replace urine examination for detection of FMLCs, but this notion has been contested [6, 17-27]. FMLCs in urine, called Bence Jones proteins, are the original tumor marker and can be reliably detected by urine protein immunofixation electrophoresis (UIFE) [14, 26].
Attempts are underway to screen the population, ostensibly to make early diagnosis of NMG, despite lack of evidence that early diagnosis improves outcomes [16, 28, 29]. Screening for MGUS is controversial, as the disorder is neither treatable nor transmissible, is not a public health burden, and causes harm by labeling a patient with a pre-malignant diagnosis without providing any benefit [29, 30]. A particularly worrisome/egregious practice has been to diagnose patients with an abnormal κ/λ ratio as having MGUS, without any evidence of monoclonality. Initially, patients with a κ/λ ratio outside the reference range of 0.25 to 1.65 were diagnosed with MGUS. Many such patients are subjected to unwarranted investigations and made to endure the stress of an unsupported pre-malignant diagnosis [30-32]. More recently, it has been proposed to raise the upper limit of the κ/λ ratio to 3.0 or 3.15 for diagnosis of kappa MGUS, still without any evidence of monoclonality [33, 34].
An abnormal κ/λ ratio is not diagnostic of a monoclonal lesion, and a normal ratio does not exclude a monoclonal lesion [6, 26]. Distortions in the ratio caused by chronic inflammation, cirrhosis, renal failure, hematopoietic stem cell transplantation, and chemotherapy have been shown to contradict some of the criteria/notions promulgated by the International Myeloma Working Group (IMWG) [35-38]. The marked preference for kappa over lambda light chain in normal and neoplastic situations has been demonstrated [6, 17, 19, 39]. NMGs have, on average, 4 - 5 times higher levels of kappa than lambda light chains [19-21, 39]. Using a normal κ/λ ratio to monitor stringent complete response has been challenged, as has been using a criterion based on the level and ratio of involved to uninvolved light chains as a myeloma defining condition [19-21, 40-47]. It has been suggested that criteria based on FLC levels ought to be light chain type-specific and should be based on the levels of monoclonal free light chains [14, 19, 26].
We posit that using abnormal κ/λ ratio to diagnose light chain MGUS is erroneous. To address this issue of correlation of FMLC in urine, SFLC levels, and diagnosis of light chain MGUS, we retrospectively examined the results of UIFE, SFLC levels, and other laboratory and clinical data over the past 14.5 years. These results are reported here.
| Materials and Methods | ▴Top |
This study was carried out at a 500+ bed tertiary care medical center affiliated with a medical school in the Southeastern United States. The study was approved by the Institutional Review Board of Augusta University. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Retrieval of UIFE data
Medical records from January 1, 2010, to June 30, 2024, were searched for UIFE results. Corresponding data on serum protein electrophoresis (SPEP), serum immunofixation electrophoresis (SIFE), SFLC, total urine protein, gamma globulin levels, creatinine levels, estimated glomerular filtration rate (eGFR), age, race, gender, and major diagnoses were recorded.
Protein electrophoreses
SPEP, SIFE, urine protein electrophoresis (UPEP), and UIFE were conducted using Helena SPIFE equipment and protocols [17, 18]. UIFE was done on random urine samples concentrated by membrane filtration [27, 44-47]. Helena antibody kits were employed for immunofixation analyses. In 2023, antisera for FLCs from Sebia (Sebia Laboratories Inc. Peachtree Corners, GA) replaced the conventional Helena anti-kappa anti-lambda antisera. Siemens analyzer and Optilite instrument, and kits from The Binding Site/Thermo Fisher Scientific (Waltham, Massachusetts, USA) were used to measure SFLC [44-50].
Subclassification of UIFE data
Results of SPEP, SIFE, and clinical data were perused to classify the specimens into four categories: UIFE-0: specimens from patients without evidence or history of MG, except solitary finding of FMLC in urine; UIFE-1: SPEP and/or SIFE and UPEP/UIFE that showed MG or there was history of MG; UIFE-3: There was evidence or history of biclonality, oligoclonal pattern, or the presence/absence of MG that could not be resolved; UIFE-4: This category was limited to three specimens from one patient with polymorphus sarcoma [47].
UIFE-0 and UIFE-1 specimens were sorted into three categories: 1) with kappa light chains, 2) with lambda light chains or 3) with both kappa and lambda light chains (biclonal lesions) or with unresolved light chain type. A small number of biclonal or unresolved cases were not processed further. Observations in UIFE-3 and UIFE-4 were not processed further. UIFE-0 and UIFE-1 kappa and lambda subgroups were further sorted by the κ/λ ratio into subgroups with ratios of ≤ 0.25, 0.26 - 0.75, 0.26 - 1.65, 1.66 - 3.0, and > 3.0. These subgroups were further sorted by presence or absence of FMLC in UIFE.
Total urine protein levels in unconcentrated urine results were subclassified into subgroups of ≤ 4.0, 5 - 10, 11 - 15, 16 - 20, 21 - 25, 26 - 30, and greater than 30 mg/dL. All serum specimens were also evaluated for difference in the concentration of “involved” and “uninvolved” light chains. Net levels for lambda and kappa light chains were designated dL and dK, respectively. Net levels of lambda and kappa light chains (dL and dK) were defined as: dL = Level of lambda free light chains minus concentration of kappa free light chains; dK = Level of kappa free light chains minus concentration of lambda free light chains.
Statistics
Data were analyzed by fitting to a multivariate logistic model using R 4.4.1.
| Results | ▴Top |
UIFE results were available for 4,998 specimens, of which 3,230 were UIFE-0 and 1,723 were UIFE-1. SFLC results and κ/λ ratios were available for 1,740 and 1,589 specimens in the UIFE-0 and UIFE-1 groups, respectively (Table 1). Race was included in the demographic data as differences among the traditional races have been noted in LCMGUS diagnosis [51]. The USA Food and Drug Administration still requires race-specific data for approval of new methods for SFLC assays.
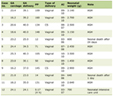 Click to view | Table 1. Categorization of Urine Immunofixation Analysis |
Lambda FMLC in urine in the UIFE-0 with respect to κ/λ ratio
In UIFE-0, seven specimens had a κ/λ ratio < 0.25 and three of the seven (42.9%) displayed FMLC on UIFE (Table 2). There were 45 specimens with κ/λ ratio from 0.26 to 0.75 and 12 of these 45 (26.7%) displayed a lambda FMLC in urine. Of the 1,688 specimens with κ/λ ratios from 0.75 to 352.79, two specimens (0.1%) displayed lambda FMLC in urine. The κ/λ ratios for the two specimens were 1.56 and 2.28. In the group of 1,140 UIFE-0 specimens with κ/λ ratio of ≤ 1.65, 16 specimens (1.4%) had lambda FMLC in urine. Only one of 600 specimens with κ/λ ratio of ≥ 1.65 had urine containing lambda FMLC (Table 2).
 Click to view | Table 2. Numbers and Percentage of Specimens in the UIFE-0 Category With Available SFLC Levels (N = 1,740) With Monoclonal Light Chains in Groups With Various Ranges of κ/λ Ratios |
The two specimens with lambda FMLC in urine with κ/λ ratio > 0.75 had polyclonal hypergammaglobulinemia. In the patient with κ/λ ratio of 1.56, the serum gamma globulin level was 2.42 g/dL, and the patient had nutritional deficiency neuropathy. The patient with κ/λ ratio of 2.28 had cirrhosis and sum of IgG, IgA, and IgM equaled 2.2 g/dL.
Additional data on the seven instructive patients with κ/λ ratio ≤ 0.25 are shown in Table 3.
 Click to view | Table 3. Additional Laboratory Data for the Seven Instructive Specimens With κ/λ Ratio of ≤ 0.25 |
In brief, about 40% of specimens with a κ/λ ratio ≤ 0.25 had FMLC in urine. Lambda FMLC was very uncommon in the group with κ/λ ratio > 0.75 and both patients in this rare group had polyclonal hypergammaglobulinemia that were likely lambda chain dominant hypergammaglobulinemias. The latter group constitutes about 5% of the cases of polyclonal hypergammaglobulinemia [6, 17].
Kappa FMLC in UIFE-0 with respect to κ/λ ratio
None of 335 specimens with κ/λ ratio ≤ 1.13 displayed kappa FMLC in urine. In 806 specimens with κ/λ ratio ≥ 1.14 and ≤ 1.65, eight (1.0%) had kappa FMLC on UIFE. Thus, in the 1,140 specimens associated with κ/λ ratio ≤ 1.65, eight (0.7%) displayed kappa FMLC (Table 2).
In 547 specimens with κ/λ ratio of 1.66 to 2.9, 13 (2.4%) had kappa FMLC in urine. There were 53 specimens with κ/λ ratio of 3 - 353 and 10 of these (18.8%) displayed kappa FMLC in urine. (Table 2). Six of seven specimens with κ/λ ratio ≥ 10 contained kappa FMLC. The one negative specimen was from a patient with cirrhosis and renal failure. Both of these pathologies can independently produce polyclonal hypergammaglobulinemia. Only four of the 46 (8.7%) specimens with κ/λ ratio ≥ 3.0 to < 10 displayed kappa FMLC in urine.
In brief, a κ/λ ratio of ≥ 10 was associated with FMLC in urine in 85.7% of the specimens in UIFE-0. None of the urine specimens with κ/λ ratio of ≤ 1.13 showed kappa FMLC in urine (Table 2). It warrants emphasis that > 80% of the urine specimens with a κ/λ ratio > 3.0 or 3.15 did not display any FMLC and according to current criteria would be labeled, erroneously, as kappa MGUS and would account for a pseudo epidemic of kappa MGUS. The results were not different with using κ/λ ratio of 3.0 or 3.15 as the upper limit of “normal”.
Results of the analysis of specimens with κ/λ ratio of 3 - 353 in UIFE-0 are shown in Table 4. Average dK levels and eGFR are significantly higher in the specimens displaying FMLC. Levels of gamma globulins are significantly higher in specimens negative by UIFE indicating that the high κ/λ ratio was the result of polyclonal hypergammaglobulinemia.
 Click to view | Table 4. Data From Patients With κ/λ Ratio 3 to 353 Regarding Average Total Urine Protein, dK, Gamma Globulins and eGFR to Contrast the Findings in Specimens Positive for Kappa Monoclonal Light Chains and Those Negative by UIFE |
Urine protein and UIFE results in UIFE 0
Only 2.1% of the UIFE-0 specimens displayed any FMLC in urine (Table 5). The prevalence of UIFE positivity showed a biphasic pattern with the highest levels in the urine with total protein concentration of 21 - 25 mg/dL. This finding has implications for the concentration of urine prior to UIFE.
 Click to view | Table 5. The Range of Total Urine Protein and the Number and Percentage of Specimens With Monoclonal Light Chains, Kappa or Lambda, in the UIFE-0 Group |
UIFE-1 group (this group had extant MG or a history of MG)
A summary of the findings from this group is presented in Table 6. Two outstanding features in the data are: 1) About 80% of the specimens from patients with κ/λ ratio < 0.25 displayed lambda FMLC in urine. 2) About 80% of the specimens from patients with κ/λ ratio > 3.0 exhibited kappa FMLC in urine. Thus, even at the extremes of κ/λ ratios, not all specimens have detectable FMLC in urine.
 Click to view | Table 6. UIFE Data for Specimens From Patients With or History of Monoclonal Gammopathy |
Additional noteworthy findings are: 1) Only about a quarter of the specimens with κ/λ ratio of 1.66 to 3.0 had kappa FMLC in urine. 2) About 4% of the specimens displayed lambda FMLC despite having a κ/λ ratio of 1.66 to 3.0. 3) In specimens with κ/λ ratio in the “normal” range of 0.26 to ≤ 1.65, about 9% and 20% of the specimens displayed kappa and lambda FMLC, respectively. Hence, even in patients with extant MG or history of MG, κ/λ ratio is not a reliable marker for the presence or absence of FMLC in urine.
UIFE-1 lambda
Descriptive data for this group are given in Tables 7-10. The various categories of specimens in the UIFE -1 lambda group are listed in Table 7. Only about one-third of the specimens displayed lambda FMLC in the face of a serum κ/λ ratio of ≤ 0.25. Overall, about 66% of the specimens displayed FMLC when light chains alone and IgG lambda MG were considered a mark of positive UIFE for lambda (Table 8). Table 9 displays the relation between dL and UIFE positivity for lambda light chains in urine. As expected, the rate of UIFE positivity for lambda monoclonal proteins was higher in specimens with low κ/λ ratio. It is noticeable that five of the specimens displayed monoclonal kappa light chains (four monoclonal kappa chains only and one with IgG kappa monoclonal protein) (Table 10). This is noted because monoclonal lambda light chains were not detected in kappa chain associated lesions. Parenthetically, the kappa monoclonal UIFE positivity in the UIFE-1 lambda group may be a manifestation of the usual kappa dominant oligoclonal pattern noted following hematopoietic stem cell transplantation.
 Click to view | Table 7. Subtypes of Specimens From Patient With Lambda Chain Associated Monoclonal Gammopathy or History of Such Lesion |
 Click to view | Table 8. Numbers and percentages of UIFE-1 Lambda Specimens Displaying Monoclonal Light Chains at Various κ/λ Ratio Categories |
 Click to view | Table 9. Numbers and Percentages of UIFE-1 Lambda Specimens Displaying Monoclonal Light Chains in Various dL Categories |
 Click to view | Table 10. Numbers and Percentages of UIFE-1 Lambda Specimens Displaying Monoclonal Light Chains in Various Categories by Total Urine Protein Levels |
When net serum lambda light chain levels, i.e., dL, were evaluated, a biphasic pattern was noted (Table 9). The highest UIFE positivity for lambda FMLC was noted with dL ≥ 3.0 and the second highest rate was with dL ≤ 1.0. Using dL did not provide any advantage over κ/λ ratio in predicting UIFE positivity for lambda FMLC.
In the UIFE-1 lambda subgroup, the proportion of specimens testing positive for lambda FMLC increased with increasing total urine protein concentration (Table 10). The number before K represents the number of specimens displaying kappa free monoclonal light chains in four specimens and one specimen with monoclonal IgG kappa.
UIFE-1 kappa
Results for UIFE-1 kappa are shown in Tables 11-14. The number of specimens in various categories are shown in Table 11. It is remarkable that in specimens with κ/λ ratio greater than 3.0, more than a third of specimens did not display kappa FMLC in urine (Table 12). It is remarkable that these patients had a kappa chain-associated MG or a history of a monoclonal lesion. The proportion of specimens with UIFE positive for kappa FMLC increased with an increasing dK value (Table 13). Similarly, the proportion of UIFE positive specimens increased with increasing total urine protein (Table 14).
 Click to view | Table 11. Subtypes of Specimens From Patient With Kappa Chain Associated Monoclonal Gammopathy or History of Such Lesion, i.e., UIFE-1 Kappa |
 Click to view | Table 12. Numbers and Percentages of UIFE-1 Kappa Specimens Displaying Monoclonal Light Chains at Various κ/λ Ratio Categories |
 Click to view | Table 13. Numbers and Percentages of UIFE-1 Kappa Specimens Displaying Monoclonal Light Chains in Various dK Categories |
 Click to view | Table 14. Numbers and Percentages of UIFE-1 Kappa Specimens Displaying Monoclonal Light Chains in Various Categories by Total Urine Protein Levels |
Results from UIFE-1 did not provide any insight to improve the interpretation of UIFE-0 specimens. The most relevant finding from review of UIFE-1 was support for the notion that κ/λ ratio by itself is not a useful marker for MG. A normal ratio does not exclude FMLC in urine and even a markedly abnormal ratio is not diagnostic of FMLC.
The regression model calculated the subject being positive (p) for UIFE based on urine protein, lambda light chain, and kappa/lambda light chain ratio as: ln(p/(1 - p)) = -4,678 - 0.0064 × U_Pr + 0.0881 × Lambda + 0.5268 × Ratio. The subject was categorized as positive for UIFE if the calculated p > 0.5. Based on this model, all the subjects were categorized as negative when lambda light chain < 49.5586 with urine protein taking an average value of 103.9281 and the kappa/lambda ratio taking an average value of 1.8621.
In multivariate logistic regression analysis, only urine protein, lambda light chain levels, and κ/λ ratio were significant. Other factors, e.g., kappa light chain level, gamma globulin, eGFR, dK, and dL were not significant and were not included in the predictive equation (Table 15).
 Click to view | Table 15. Regression Report for UIFE-0 |
| Discussion | ▴Top |
The findings and interpretations of this study, with respect to the College of American Pathologists “Expert Panel “on the Laboratory Detection and Initial Diagnosis of Monoclonal Gammopathies” recommendations are addressed first [38].
The primary recommendation of the guidelines was to include SFLC measurements as screening tests for MG. The authors also recognized that a normal serum κ/λ ratio does not exclude MG and an abnormal ratio is not diagnostic of MG [6, 17, 18].
Some of the items in this landmark publication are somewhat outdated, as newer information became available after the drafting of the Guidelines Statement. For example, the entity of LCPMM was described after the guidelines were drafted. Additional relevant publications, since the drafting of the statement and relevant to the issues addressed are cited [11-14, 26, 43-56].
The nine recommendations are addressed individually, in the context of light chain monoclonal gammopathy of undetermined significance (LCMGUS) diagnosis. A tenth recommendation is proposed.
Guideline 1 recommends using SPEP and SFLC in screening for M proteins. It does not address light chain disorders, and as shown in the current study, SFLC is sorely lacking in both sensitivity and specificity for diagnosing LCMGUS [6, 17, 18, 43-47]. The FLC-modified SIFE method detects free monoclonal light chains and has the sensitivity of mass spectrometry and far exceeds that of MASS FIX MALDI [43-47]. FLC-modified SIFE method has been automated [45]. The entity of light chain predominant MG was not known to the drafters of the guidelines [11-14].
Guideline 2 recommends using SIFE to confirm the suspicion of an M protein from SPEP. This is not applicable to LCMGUS as such lesions rarely produce a peak on SPEP [44].
Guideline 3 recommends verifying an abnormal SFLC with SIFE or another method of similar sensitivity. We emphasize that the FLC-modified SIFE has greater sensitivity than SIFE for free monoclonal light chains and should be used to diagnose LCMGUS. This information was not available to the Expert Panel at the time of drafting of the report [43-47].
Guideline 4 advocates the use of SPEP, SFLC, SIFE, and UIFE in evaluation for amyloidosis. This is reasonable but not relevant to the diagnosis of LCMGUS. However, the FLC-UIFE method improved the sensitivity of UIFE by 18% [46].
Guideline 5 relates to the limited utility of heavy/light chain (HLC) reagent, which also is not relevant to the diagnosis of LCMGUS. The test in question would not be useful for LCMGUS. In fact, this test is rarely used in clinical laboratories.
Guideline 6 recommends against testing for total light chains and is not relevant to the current issue.
Guideline 7 addresses peaks outside the gamma region, where cognate protein interference limits detection. This matter is not relevant to LCMGUS [47-49].
Guideline 8 recommends reporting actual light chain concentration figures and is reasonable. However, as noted earlier, SFLC is neither a sensitive nor a specific test for LCMGUS [6, 17, 18, 42-45].
Guideline 9 addresses the additional risk with diagnosis of IgM isotype. It is not relevant to the diagnosis or prognosis of LCMGUS.
We propose that guideline 10 be added and should state: A diagnosis of MG should not be made without unequivocal evidence of monoclonality. An abnormal κ/λ ratio is not diagnostic of monoclonality [6, 17, 18, 42-45].
Quantification of SFLC has been advocated as a surrogate/replacement for UIFE. In a hallmark study, blood donors and healthy residents of a single northern US county were used to derive the reference range [23, 24]. The whole range rather than the central 95% of the range was used, and a ratio of κ/λ SFLC concentration of 0.25 to 1.65 was recommended as the normal range. This was taken to interpret that patients with a κ/λ ratio outside this range had MG. Currently, in standard clinical practice, practitioners diagnose patients with a κ/λ ratio outside this range as having MGUS, without documenting any evidence of monoclonality. When the same reference range was applied to patients presenting to a tertiary care medical center, more than a third of the patients had an abnormal serum κ/λ ratio, without any evidence of MG [6, 17].
In patients with recent diagnosis of LCMM, the presence of an abnormal serum κ/λ ratio in all patients was cited as one criterion for replacement of UIFE with SFLC [25]. However, these authors also documented that urine contained FMLC in all cases, even though the authors did not concentrate the urine before conducting UIFE. This publication cited the superiority of a κ/λ ratio because following treatment the ratio was abnormal more often than the presence of FMLC in urine. However, as a caveat, there was no independent proof of monoclonality of light chains in serum or urine with an abnormal serum κ/λ ratio [25, 40]. It was later shown that following autologous stem cell transplantation (ASCT), more patients with lambda chain associated MM have a kappa-dominant abnormal κ/λ ratio rather than a lambda-dominant abnormal κ/λ ratio, thus supporting the notion that the abnormal κ/λ ratio seen following treatment may not be due to lesional monoclonal light chains [40]. The oligoclonal pattern seen following ASCT usually produces a kappa-dominant abnormal κ/λ ratio irrespective of the original light chain with the MM lesion. This preference for kappa oligoclones results in a large number of false positive kappa-dominant κ/λ ratios causing a misdiagnosis of lack of stringent complete response [35, 40]. Many patients with MM, especially following treatment, may have polyclonal hypergammaglobulinemia [40]. It has been reported that about 25% of lambda chain-associated MM may have lambda FMLC in urine with a normal serum κ/λ ratio [40]. The κ/λ ratio is tilted towards normality due the presence of excessive polyclonal kappa light chains despite the presence of monoclonal lambda light chains [17, 40].
Another potential confounder has been that the reported reactivity of the antiserum to free kappa light chains in the diagnostic assay used in these reports (Freelite® from The Binding Site) has shown temporal drift and now results in higher kappa SFLC levels than was observed about 20 years ago [52]. The conventional recommendation that patients with a κ/λ ratio greater than 1.65 should be diagnosed with kappa MGUS has been revised such that the ratio is recommended to be raised to 3.0. However, as in the previous recommendation, there was no documented correlation of κ/λ ratio ≥ 3.0 with the presence of any monoclonal immunoglobulins [33]. Similarly, the upper limit of ratio at 3.15 proposed by iStopMM is not supported by independent evidence of monoclonality [34]. A further confounder in this assay is that antiserum reactivity may just as well drift in the opposite direction requiring further revision of this unreliable standard. A small but appreciable number of patients with IgG or IgA lambda MMs do not secrete enough excess lambda FMLC to render the κ/λ ratio abnormal [18].
In clinical practice, a low κ/λ ratio has not been equated with lambda MGUS with the same rigor as applied to a high κ/λ ratio being labeled kappa MGUS. The results from the UIFE-0 group in the current study are instructive in further documenting that an abnormal κ/λ ratio is not diagnostic of monoclonality. The lack of monoclonal lambda light chains in urine with a κ/λ ratio as low as 0.11 can be explained by polyclonal hypergammaglobulinemia with high levels of polyclonal lambda light chains producing a low κ/λ ratio. It has been observed that more than 50% of the patients with polyclonal hypergammaglobulinemia have an abnormal κ/λ ratio. In about 95% of these cases, the abnormal κ/λ ratio is kappa-dominant; however, about 5% of the subjects have a ratio that favors excessive lambda chains [6, 17]. Thus, in the patient with a κ/λ ratio of 0.11 and gamma globulin level of 3.54 g/dL (Table 3), it is probable that the polyclonal hypergammaglobulinemia was primarily due to polyclonal IgG lambda and the high level of lambda SFLCs was due to polyclonal free lambda light chains [5, 6]. The same explanation also could be applicable to the patient with a κ/λ ratio of 0.12. Other plausible reasons for negative UIFE in patients with a low κ/λ ratio include lack of adequate concentration of urine. It is recommended in standard laboratory practice that urine be concentrated 100-fold for UIFE; however, this is often not practical [27, 46]. Explanations for additional discrepant results are listed in the legend for Table 3. Discrepant results could also be due to variability in the reactivity of antisera to FMLC [44-50]. Bradwell observed lack of reactivity of the antiserum to monoclonal kappa light chains from one patient [22]. Variations in the reactivities of antisera to FLCs from different vendors have been reported [44-46].
The other discrepant results for lambda FMLC in UIFE were the observation of monoclonal lambda light chains in specimens from patients with high κ/λ ratios. Illustrative of this, two patients with κ/λ ratios > 1.0 both had polyclonal hypergammaglobulinemia, and the high concentration of free polyclonal kappa light chains was likely producing a kappa-dominant ratio while the monoclonal lambda light chains were still detectable in urine [17].
The controversial recommendation of labeling patients with a high κ/λ ratio as having kappa MGUS is effectively challenged by the findings of this study. Fewer than 1.0% of urine specimens from patients with a κ/λ ratio ≤ 1.65 displayed kappa FMLC in urine. At the same time, the proportion showing positive results for kappa FMLC with κ/λ ratio 1.66 to 2.99 was at a less than impressive 2.4%. This would call into serious question that this level is a threshold to diagnose kappa MGUS. In diagnosis-naive patients with a κ/λ ratio of 3 to 10, only about 9% of the patients displayed kappa FMLC. When taken to the extreme, only 18.8% of patients with a κ/λ ratio of 3.0 and higher (to a κ/λ ratio of 353) displayed monoclonal kappa light chains in urine. Thus, even the recommendation to implement a κ/λ ratio level ≥ 3.0, or 3.15 being diagnostic of kappa MGUS would generate more than 80% false positives. The findings in the UIFE-1 group support our contention that even in patients with the known presence of MG, the κ/λ ratio is an unreliable marker for monoclonality. In effect, employing the current guidelines would create a pseudo epidemic of MGUS that does not merit clinical evaluation.
The lack of kappa FMLC in the presence of a high κ/λ ratio is easier to explain as most patients with polyclonal hypergammaglobulinemia produce an excess of free polyclonal kappa light chains, resulting in a high κ/λ ratio without there being any FMLC. One extreme patient with a κ/λ ratio of 11.53 and a lack of kappa FMLC had both polyclonal hypergammaglobulinemia and chronic renal failure. These two pathologies are associated with high levels of polyclonal kappa light chains.
Additional factors potentially accounting for the discrepant results noted in the UIFE-0 group for lambda light chains are also applicable to kappa light chains, i.e., false negative results could be due to 1) lack of adequate concentration of urine; 2) diminished accessibility of FLCs in IgA monoclonal lesions; and 3) variability in antibody reactivity from different vendors [44, 45, 50, 55, 56]. The discrepancy of high SFLC and lack of FMLC in urine could not be fully addressed due to the lack of availability of antisera from The Binding Site. When a discrepancy is seen with high serum free kappa light chain levels and absence of any other evidence for FMLC using antisera from other vendors, a doubt remains if the discrepancy is due to the variation in the reactivities of sera from The Binding Site and other vendors.
The current study has the usual limitations that the analyses originate from a single institution and that this is a retrospective observational review. A prospective study evaluating the comparable utility of UIFE and SFLC assay may be useful if discrepant results could be further evaluated by antibodies from multiple sources, using more sensitive techniques, e.g., FLC-modified SIFE, FLC-UIFE, and mass spectrometry. An appropriate standardized level of urine concentration with reference to urine protein needs to be determined. With additional analyses, a more conservative recommendation regarding κ/λ ratios in MGUS diagnosis may benefit the clinical laboratory and clinicians alike. Incorporating additional clinical parameters may be of benefit in elucidating a more consistent diagnosis. For instance, incorporating urine protein levels together with lambda light chain levels and κ/λ ratio renders a regression equation (ln(p/(1 - p)) = -4,678 - 0.0064 × U_Pr + 0.0881 × Lambda + 0.5268 × Ratio) with a predictive accuracy of 97%. More independent datasets are needed to test the validity and improve the reliability of the prediction model.
Conclusions
The main conclusion of the study is that more than 80% of serum specimens with κ/λ ratio ≥ 3.0 or 3.15 have no evidence of monoclonality. Using the standard of κ/λ ratio ≥ 3.0 or 3.15 as a diagnostic marker for kappa MGUS would engender an unacceptable level of false-positive rate of > 80% for kappa MGUS, resulting in a pseudo epidemic of this “disorder”. Diagnosis of an MGUS ought not be made without unequivocal evidence of monoclonality.
Learning points
An abnormal serum κ/λ ratio is not diagnostic of monoclonality.
A normal serum κ/λ ratio does not exclude monoclonality.
UIFE can be effectively carried out on a random urine specimen, and a 24-h urine is not required.
A 100-fold concentration of urine, when feasible, is strongly recommended for UIFE.
SFLC assay and κ/λ ratio are not reliable surrogates for UIFE for demonstration of monoclonality.
SFLC assay does not meet the criteria to constitute an effective screening test for MGUS. At a minimum, concurrent UIFE ought to be a part of the screening algorithm for monoclonal lesions.
Polyclonal hypergammaglobulinemia impedes the utility of abnormal serum κ/λ ratio. In about 95% of the cases of polyclonal hypergammaglobulinemia and abnormal κ/λ ratio, the ratio is kappa-dominant, and in about 5% of instances, it may result in a lambda-dominant abnormal κ/λ ratio, without any monoclonal lesion.
Optimal implementation of UIFE warrants using antisera to FLCs, in addition to proper urine concentration. FLC antibodies have about 20% higher sensitivity than conventional UIFE.
Acknowledgments
We are grateful to Dr. Rhea-Beth Markowitz for editing the manuscript.
Financial Disclosure
No external funding was received for this study.
Conflict of Interest
GS served as a consultant to Sebia Inc., Diazyme Laboratories, Helena Laboratories and Beckman Coulter.
Informed Consent
Requirement of consent was waived for this retrospective review of records.
Author Contributions
GS conceived the study, collected, analyzed the data, and drafted the manuscript. HX conducted the statistical analysis. RB contributed to data analysis and revised the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ASCT: autologous stem cell transplantation; eGFR: estimated glomerular filtration rate; FMLC: free monoclonal light chain; IMWG: International Myeloma Working Group; LC: light chain; LCPMM: light chain predominant multiple myeloma; MG: monoclonal gammopathy; MGUS: monoclonal gammopathy of undetermined significance; MM: multiple myeloma; NMG: neoplastic monoclonal gammopathy; SFLC: serum free light chain; SMM: smoldering multiple myeloma; UIFE: urine protein immunofixation electrophoresis; UIFE-0: specimens from patients without evidence or history of MG, except solitary finding of FMLC in urine; UIFE-1: SPEP and/or SIFE and UPEP/UIFE showed MG or there was history of MG; UIFE-3: There was evidence or history of biclonality, oligoclonal pattern, or the presence/absence of MG could not be resolved; UIFE-4: This category was limited to three specimens from one patient with polymorphus sarcoma; UPEP: urine protein electrophoresis; Net level of lambda and kappa light chains (dL and dK): i.e., dL = Level of lambda free light chains minus concentration of kappa free light chains. dK= Level of kappa free light chains minus concentration of lambda free light chains
| References | ▴Top |
- Tate JR. The paraprotein - an enduring biomarker. Clin Biochem Rev. 2019;40(1):5-22.
pubmed - Genzen JR, Murray DL, Abel G, Meng QH, Baltaro RJ, Rhoads DD, Delgado JC, et al. Screening and diagnosis of monoclonal gammopathies: an international survey of laboratory practice. Arch Pathol Lab Med. 2018;142(4):507-515.
doi pubmed - Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962-2972.
doi pubmed - Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241-249.
doi pubmed - Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
doi pubmed - Singh G. Serum and urine protein electrophoresis and serum-free light chain assays in the diagnosis and monitoring of monoclonal gammopathies. J Appl Lab Med. 2020;5(6):1358-1371.
doi pubmed - Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, et al. SEER Cancer Statistics Review, 1975-2013. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
- Islami F, Ward EM, Sung H, Cronin KA, Tangka FKL, Sherman RL, Zhao J, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. J Natl Cancer Inst. 2021;113(12):1648-1669.
doi pubmed - Landgren O, Rajkumar SV. New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clin Cancer Res. 2016;22(22):5428-5433.
doi pubmed - Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, Medvedova E, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387(2):132-147.
doi pubmed - Singh G, Xu H. Light chain predominant intact immunoglobulin monoclonal gammopathy disorders: shorter survival in light chain predominant multiple myelomas. Lab Med. 2021;52(4):390-398.
doi pubmed - Singh G, Savage NM, Jillella AP, Bollag RJ. Light chain-predominant multiple myeloma subgroup: impaired renal function correlates with decreased survival. Lab Med. 2022;53(2):145-148.
doi pubmed - Jin Y, Savage NM, Bollag RJ, Xu H, Singh G. Light chain multiple myeloma: high serum free light chain concentrations portend renal damage and poorer survival. J Appl Lab Med. 2021;6(6):1592-1600.
doi pubmed - Singh G, Xu H, Bollag RJ. Monoclonal light chains in multiple myeloma: The sinister immunoglobulins. International Journal of Pathology and Clinical Research. 2022;8:134-142.
- Schroeder HW, Jr., Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41-52.
doi pubmed - Uljon SN, Richardson PG, Schur PH, Anderson KC, Tanasijevic MJ, Lindeman NI. Serial serum free light chain measurements do not detect changes in disease status earlier than electrophoretic M-spike measurements in patients with intact immunoglobulin myeloma. Clin Chim Acta. 2011;412(7-8):562-568.
doi pubmed - Singh G. Serum free light chain assay and kappa/lambda ratio performance in patients without monoclonal gammopathies: high false-positive rate. Am J Clin Pathol. 2016;146(2):207-214.
doi pubmed - Singh G. Serum Free Light chain assay and kappa/lambda ratio: performance in patients with monoclonal gammopathy-high false negative rate for kappa/lambda ratio. J Clin Med Res. 2017;9(1):46-57.
doi pubmed - Lee WS, Singh G. Serum free light chains in neoplastic monoclonal gammopathies: relative under-detection of lambda dominant kappa/lambda ratio, and underproduction of free lambda light chains, as compared to kappa light chains, in patients with neoplastic monoclonal gammopathies. J Clin Med Res. 2018;10(7):562-569.
doi pubmed - Singh G. Concentrations of serum free light chains in kappa and lambda lesions in light-chain myelomas. Lab Med. 2019;50(2):189-193.
doi pubmed - Lee WS, Singh G. Serum Free Light Chain Assay in Monoclonal Gammopathic Manifestations. Lab Med. 2019;50(4):381-389.
doi pubmed - Bradwell AR. 2015 Serum free light chain analysis, plus Hevylite: the Binding Site Group Ltd., 7th ed.
- Katzmann JA, Dispenzieri A, Kyle RA, Snyder MR, Plevak MF, Larson DR, Abraham RS, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc. 2006;81(12):1575-1578.
doi pubmed - Katzmann JA. Screening panels for monoclonal gammopathies: time to change. Clin Biochem Rev. 2009;30(3):105-111.
pubmed - Dejoie T, Corre J, Caillon H, Hulin C, Perrot A, Caillot D, Boyle E, et al. Serum free light chains, not urine specimens, should be used to evaluate response in light-chain multiple myeloma. Blood. 2016;128(25):2941-2948.
doi pubmed - Singh G. Free monoclonal immunoglobulin light chains in serum and urine. 21st Century Pathology. 2023;3:143-154.
- Shaheen SP, Levinson SS. Serum free light chain analysis may miss monoclonal light chains that urine immunofixation electrophoreses would detect. Clin Chim Acta. 2009;406(1-2):162-166.
doi pubmed - Goldschmidt N, Zamir L, Poperno A, Kahan NR, Paltiel O. Presenting Signs of Multiple Myeloma and the Effect of Diagnostic Delay on the Prognosis. J Am Board Fam Med. 2016;29(6):702-709.
doi pubmed - Visram A, Larson D, Norman A, Dispenzieri A, Murray D, Kyle R, Rajkumar SV, et al. Comparison of progression risk of monoclonal gammopathy of undetermined significance by method of detection. Blood. 2025;145(3):325-333.
doi pubmed - Maatouk I, He S, Hummel M, Hemmer S, Hillengass M, Goldschmidt H, Hartmann M, et al. Patients with precursor disease exhibit similar psychological distress and mental HRQOL as patients with active myeloma. Blood Cancer J. 2019;9(2):9.
doi pubmed - Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Therneau TM, Colby CL, Clark RJ, et al. Use of nonclonal serum immunoglobulin free light chains to predict overall survival in the general population. Mayo Clin Proc. 2012;87(6):517-523.
doi pubmed - Kaur J, Valisekka SS, Hameed M, Bandi PS, Varma S, Onwughalu CJ, Ibrahim H, et al. Monoclonal gammopathy of undetermined significance: a comprehensive review. Clin Lymphoma Myeloma Leuk. 2023;23(5):e195-e212.
doi pubmed - Rozenova K, Willrich M, Snyder M, Dasari S, Kourelis T, Rajkumar SV, Kumar S, et al. Kappa free light chain drift prompts the need for a new upper limit of normal free light chain ratio to avoid an epidemic of kappa light chain monoclonal gammopathy of undermined significance. J Appl Lab Med. 2023;8(4):742-750.
doi pubmed - Oskarsson J, Rognvaldsson S, Thorsteinsdottir S, Long TE, Olafsson A, Eythorsson E, Jonsson A, et al. The significance of free light-chain ratio in light-chain monoclonal gammopathy of undetermined significance: a flow cytometry sub-study of the iStopMM screening study. Blood Cancer J. 2024;14(1):221.
doi pubmed - Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346.
doi pubmed - Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
doi pubmed - Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kroger N, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-1127.
doi pubmed - Keren DF, Bocsi G, Billman BL, Etzell J, Faix JD, Kumar S, Lipe B, et al. Laboratory detection and initial diagnosis of monoclonal gammopathies. Arch Pathol Lab Med. 2022;146(5):575-590.
doi pubmed - Garcia de Veas Silva JL, Bermudo Guitarte C, Menendez Valladares P, Rojas Noboa JC, Kestler K, Duro Millan R. Prognostic value of serum free light chains measurements in multiple myeloma patients. PLoS One. 2016;11(11):e0166841.
doi pubmed - Singh G. Oligoclonal pattern/abnormal protein bands in post-treatment plasma cell myeloma patients: implications for protein electrophoresis and serum free light chain assay results. J Clin Med Res. 2017;9(8):671-679.
doi pubmed - Pelzer BW, Arendt M, Moebus S, Eisele L, Jockel KH, Duhrsen U, Durig J, et al. Light chain monoclonal gammopathy of undetermined significance is characterized by a high disappearance rate and low risk of progression on longitudinal analysis. Ann Hematol. 2018;97(8):1463-1469.
doi pubmed - Brigden ML, Neal ED, McNeely MD, Hoag GN. The optimum urine collections for the detection and monitoring of Bence Jones proteinuria. Am J Clin Pathol. 1990;93(5):689-693.
doi pubmed - Singh G. Monoclonal gammopathy. Br J Haematol. 2025;206(6):1857-1858.
doi pubmed - Wilhite D, Arfa A, Cotter T, Savage NM, Bollag RJ, Singh G. Multiple myeloma: Detection of free monoclonal light chains by modified immunofixation electrophoresis with antisera against free light chains. Pract Lab Med. 2021;27:e00256.
doi pubmed - Singh G, Saldana EJ, Spencer J, Bollag RJ. Automated detection of free monoclonal light chains by enhanced-sensitivity modified immunofixation electrophoresis with antisera against free light chains. Lab Med. 2025.
doi pubmed - Singh G, Arinze N, Manthei DM, Plapp FV, Bollag RJ. Urine protein immunofixation electrophoresis: free light chain urine immunofixation electrophoresis is more sensitive than conventional assays for detecting monoclonal light chains and could serve as a marker of minimal residual disease. Lab Med. 2023;54(5):527-533.
doi pubmed - Singh G, Bollag R. Quantification by ultrafiltration and immunofixation electrophoresis testing for monoclonal serum free light chains. Lab Med. 2020;51(6):592-600.
doi pubmed - Clavijo A, Fallaw D, Coule P, Singh G. Communication of critical laboratory values: optimization of the process through secure messaging. Lab Med. 2020;51(1):e6-e11.
doi pubmed - Omar N, Madwani K, Moideen P, Manthei DM, Keren DF, Singh G. Accurate quantification of monoclonal immunoglobulins migrating in the beta region on protein electrophoresis. Lab Med. 2022;53(2):138-144.
doi pubmed - Singh G, Cotter T, Ye Mon M, Xu H, Bollag RJ. Quantification of free immunoglobulin light chains in urine. J Appl Lab Med. 2023;8(6):1101-1114.
doi pubmed - Bertamini L, Alberge JB, Lee DJ, El-Khoury H, Kim S, Fleming G, Murphy C, et al. Serum free light chains in a racially diverse population including African Americans and populations from South Africa. Blood. 2025;145(8):840-849.
doi pubmed - Griffiths M, Schneider RJ, Kulasingam V. Serum free light chain and drift: calibrator adjustment needed? J Appl Lab Med. 2024;9(2):394-396.
doi pubmed - Ye Mon M, Ufondu O, Mortley S, Bollag RJ, Singh G. Urine immunofixation electrophoresis for diagnosis of monoclonal gammopathy: evaluation of methods for urine concentration. J Appl Lab Med. 2024;9(2):350-356.
doi pubmed - Cotten SW, Shajani-Yi Z, Cervinski MA, Voorhees T, Tuchman SA, Korpi-Steiner N. Reference intervals and diagnostic ranges for serum free kappa and free lambda immunoglobulin light chains vary by instrument platform: Implications for classification of patient results in a multi-center study. Clin Biochem. 2018;58:100-107.
doi pubmed - Singh G, Whitaker BM, Wu AHB, Xu H, Bollag RJ. Serum free light chain quantification testing: comparison of two methods for disease monitoring. J Appl Lab Med. 2022;7(6):1290-1301.
doi pubmed - Nwogbo OV, Jin Y, Sliker T, Wilhite D, Singh G. Analysis of multiple bands on serum protein immunofixation electrophoresis: challenge in interpretation of clonality in a patient with light chain-predominant multiple myeloma. Lab Med. 2021;52(5):503-508.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.