Figures
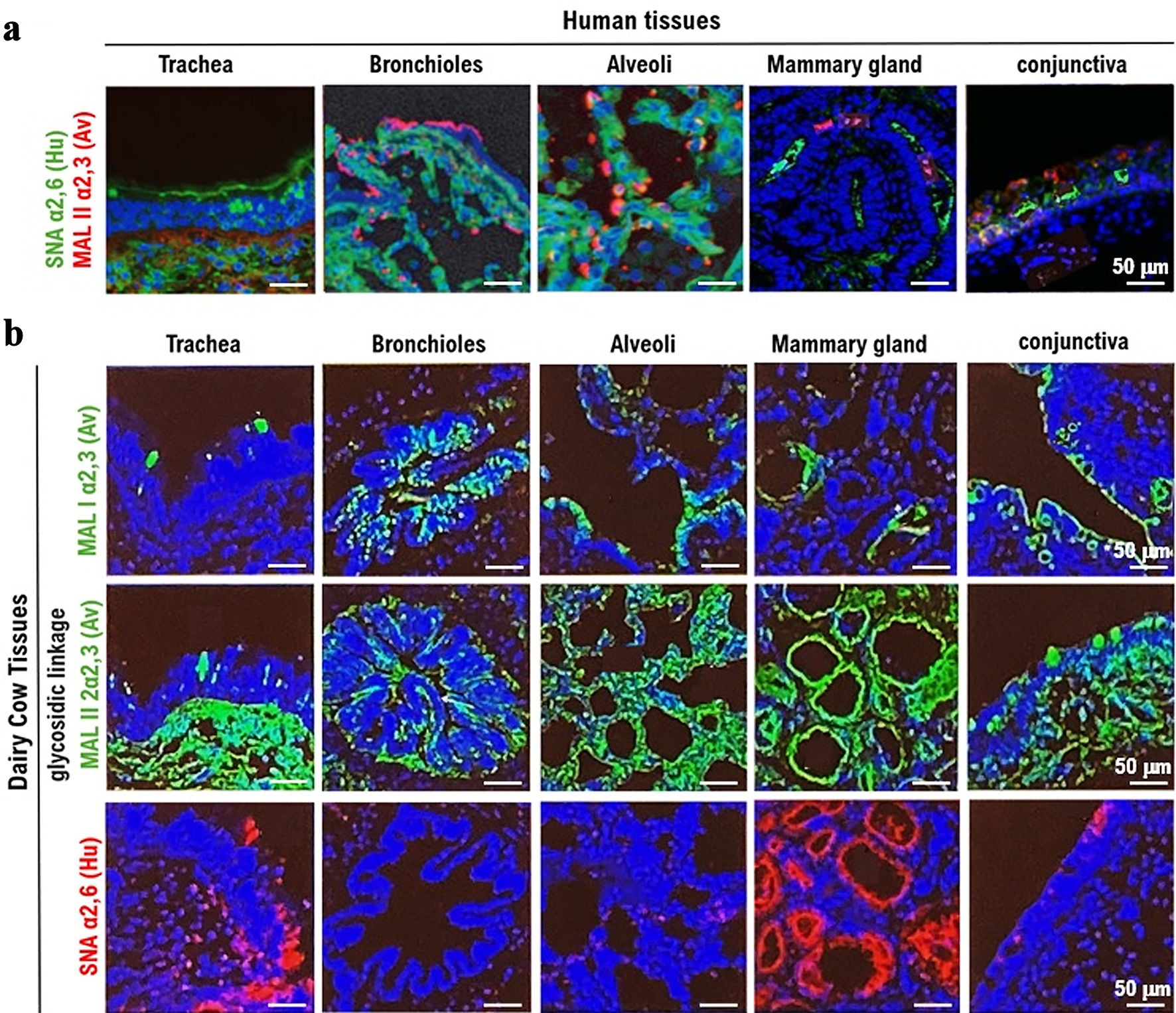
Figure 1. Expression of the α2,3SA receptor for HPAI/H5N1 and the α2,6SA receptor for human influenza virus in respiratory, mammary gland, and conjunctival tissues obtained from humans and dairy cows. (a) The expression of the HPAI/H5N1 virus receptor α2,3SA (red) and human influenza virus receptor α2,6SA (green) in human respiratory (trachea, bronchioles, and alveoli), mammary gland, and conjunctival tissues. α2,6SA (green) is expressed in all tissues, particularly in the bronchioles and alveoli. However, α2,6SA was weakly expressed in human mammary gland and conjunctival tissues. Green: reaction with Sambucus nigra lectin, indicating the presence of sialic acid linked to galactose by an α2,6SA-linkage (SA_2,6Gal). Red: reaction with Maackia amurensis lectin, indicating the presence of SA_2,3Gal. Cells were counterstained with DAPI. All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. The experiments were repeatedly performed with mammary tissues from five adult women. (b) The expression of α2,3SA (green) and α2,6SA (red) in respiratory (trachea, bronchioles, and alveoli), mammary gland, and conjunctival tissues obtained from dairy cows. α2,6SA (red) is strongly expressed in the mucosal epithelial tissue of the trachea and mammary gland tissue cells. The expression of α2,6SA was not detected in the bronchioles, alveoli, or conjunctiva. MAL I lectin (red) or MAL II lectin (red) is an antagonist of α2,3SA, while SMA is an antagonist of α2,6SA. All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. The experiments were repeatedly performed with mammary tissues from five dairy cows. HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; DAPI: 4',6-diamidino-2-phenylindole.
Figure 2. Expression of the α2,3SA, and α2.SA6 in respiratory tissues obtained from lions. Like coronavirus disease 2019 (COVID-19), there is a possibility that lions kept in zoos could in the future become infected with HPAI infectious disease through zookeepers infected with HPAI/H5N1. Therefore, we confirmed by immunohistochemical staining the expression of α2,3SA, and α2.SA6, which are the host’s receptors for H1N1 and H5N1 in the respiratory tissues (bronchioles and alveoli) obtained from lions. Bronchioles and alveoli tissues were incubated with SMA lectin (red) for 12 h at 4 °C. The tissues were then washed with phosphate-buffered saline (PBS) and observed under a confocal laser microscope. The expression of α2,6SA (red) was observed in the bronchioles and alveoli. Respiratory tissues (bronchioles, alveoli) from lions were also incubated with MAL I lectin (red) or MAL II lectin (red) for 12 h at 4 °C. The tissues were then washed with PBS and observed under a confocal laser microscope. α2,3SA (red) was also expressed in the respiratory tissues (bronchioles and alveoli). All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. The experiments were repeatedly performed with mammary tissues from five dairy cows. hHA: HA protein derived from human influenza virus (H1N1); aHA: HA protein derived from high pathogenic avian influenza virus (H5N1); HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; HA: hemagglutinin.
Figure 3. Comparison of the expression of α2,6SA and α2,3SA among human, dairy cow, and lion tissues. (a) The expression levels of host receptors α2,3SA and α2,6SA were quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan). The expression of α2,3SA was higher than that of α2,6SA in all human or dairy cow tissues. Furthermore, the expression of α2,3SA was higher than that of α2,6in all dairy cow tissues. (b) The expression of α2,6SA was higher than that of α2,3SA in the bronchiolar and alveolar tissues obtained from lions. α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid.
Figure 4. Binding activity of HPAI/H5N1 variants isolated from infected dairy cows to the receptor (α2,6SA) for the human influenza virus. We investigated the binding affinity/recognition of the HPAI/H5N1) variant (called as bovine H5) isolated from infected dairy cows to the α2,6SA or α2,3SA receptors using an analog of the α2,6SA receptor (LSTc) or an analog of the α2,3SA receptor (LSTa) using in silico analysis. HA of bovine H5 recognized and bound to an analog of the α2,6SA receptor (LSTc) or an analog of the α2,3SA receptor (LSTa) (a, d). In silico analysis showed strong binding affinity/recognition between the positive control Indonesia H5 (Indonesia H5 R192) and the α2,6SA or α2,3SA receptors (b, e). Indonesia H5 mutant showed somewhat weak binding (affinity) to the α2,6SA receptor or α2,3SA receptor (c, f). Indonesia H5 R192 variant is called as Indonesian H5 mut. HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; HA: hemagglutinin.
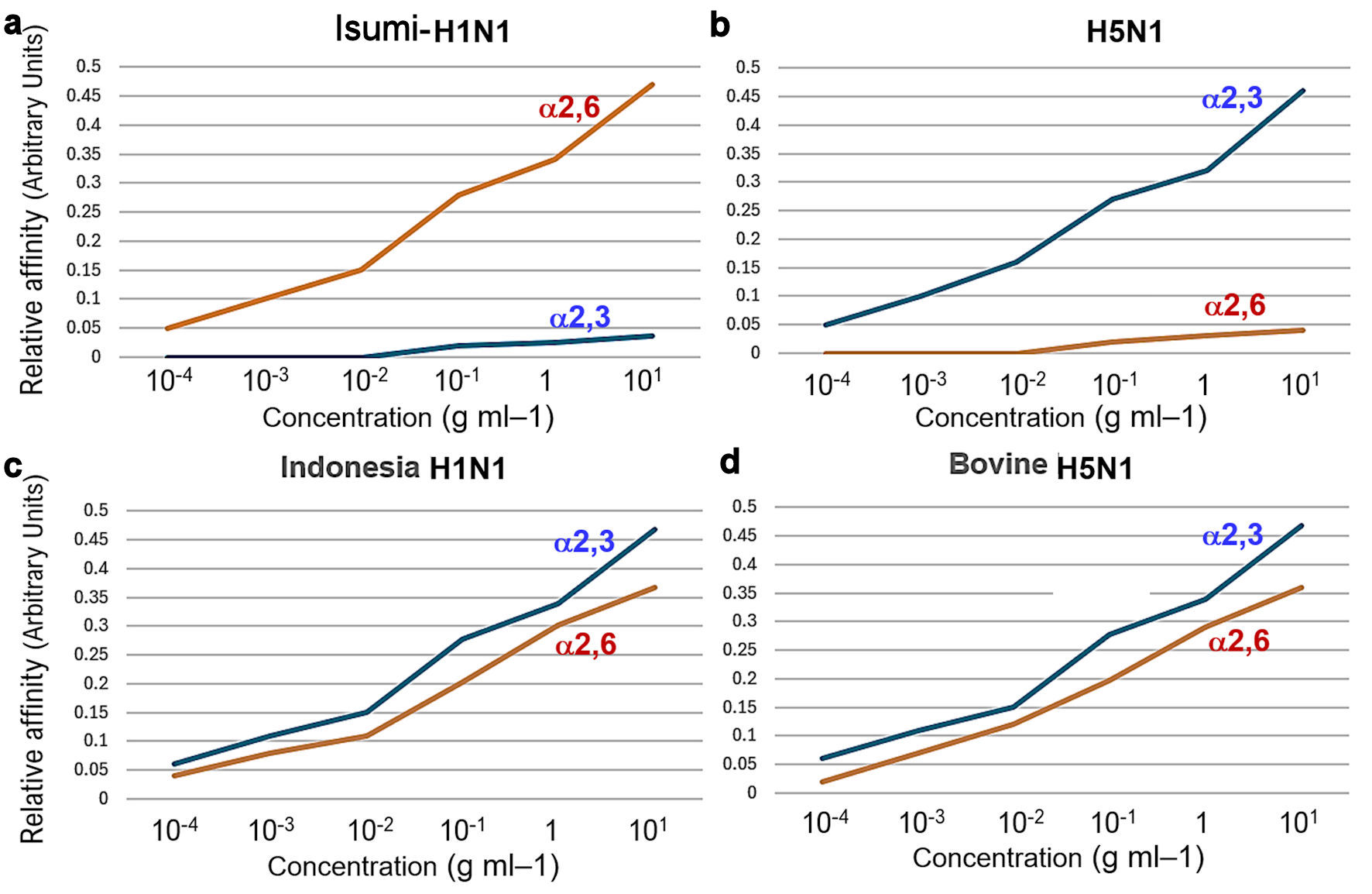
Figure 5. To further test the receptor specificity of bovine H5N1, we measured the binding affinity/recognition of recombinant bovine HA to either α2,3- or α2,6-linked sialic acid using established assays with either α2,3- or α2,6-linked sialylglycopolymers. Isumi-H1N1 virus HA showed a clear preference for α2,6-linked sialic acid, whereas HPAI (H5N1) virus HA also showed a clear preference for α2,3-linked sialic acid (a, b). As expected, Indonesia-human H5N1 R192 virus HA showed preference for α2,3-linked sialic acid, whereas bovine HPAI (H5N1) virus HA also showed preference for α2,6-linked sialic acid (c, d). HPAI: highly pathogenic avian influenza virus; HA: hemagglutinin.
Figure 6. Comparison of the expression of host receptors (α2,3SA) for HPAI/H5N1 viruses in human and dairy cow mammary tissues. (a) Paraffin-embedded human and bovine mammary tissue slices were incubated with recombinant H5N1 HA protein-His Tag for 12 h at 4 °C. Then, the labeled anti-His Tag antibody was added to the treated human and bovine mammary tissue slices and incubated for 12 h at 4 °C, and nuclear staining was performed with DAPI. We also incubated paraffin-embedded human tissue slices with anti-avian α2,3 antibody at 4 °C for 12 h and then stained the nuclei with DAPI. The stained images of each tissue section were photographed using a confocal laser microscope (LEICA SP8 FALCON). We then saved each image as a file and merged the three image files (DAPI, HA, α2,3SA). All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. (b) The expression levels of host receptor α2,3SA is quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan). The quantified expression levels of host receptor α2,3SA is then plotted on a graph. *P < 0.005. All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; DAPI: 4',6-diamidino-2-phenylindole; HA: hemagglutinin.





