| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://cmmr.elmerpub.com |
Letter to the Editor
Volume 000, Number 000, October 2025, pages 000-000
Unraveling One-Carbon Metabolism in Metabolic Dysfunction-Associated Steatotic Liver Disease: Semaglutide Turns Metabolic Stress Into Metabolic Resilience
Amedeo Lonardoa, c, d , Ralf Weiskirchenb, c, d
aDepartment of Internal Medicine, Azienda Ospedaliero-Universitaria of Modena (-2023), Modena 41100, Italy
bInstitute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC), RWTH University Hospital Aachen, Aachen D-52074, Germany
cBoth authors contributed equally to this work.
dCorresponding Authors: Amedeo Lonardo, Department of Internal Medicine, Azienda Ospedaliero-Universitaria of Modena (-2023), Modena 41100, Italy; Ralf Weiskirchen, Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC), RWTH University Hospital Aachen, Aachen D-52074, Germany
Manuscript submitted September 12, 2025, accepted September 18, 2025, published online October 10, 2025
Short title: Unraveling OCM in MASLD
doi: https://doi.org/10.14740/cmmr107
| Letter to the Editor | ▴Top |
Introduction
What do methotrexate, sulfamethoxazole, supplementation of folic acid in pregnancy, and certain macrocytic anemias have in common? The answer is simple: they all highlight the crucial importance of folic acid in regulating biological processes across bacteria to mammalian cells, both in prenatal life and in human adults [1]. For example, methotrexate, a folate analogue, is used as a chemotherapeutic agent and in autoimmune disorders because, by inhibiting key enzymes involved in nucleic acid biosynthesis, this drug helps control the rapid turnover seen in both cancerous and inflammatory cells [2, 3]. Sulfamethoxazole, a sulfonamide agent, blocks the production of active folic acid in susceptible organisms, including many gram-positive aerobic cocci, some gram-negative aerobic bacilli, and certain opportunistic infection-causing agents [4]. Folic acid deficiency (FAD), which can occur because of insufficient dietary intake, intestinal malabsorption, increased physiological demand, or certain medications [5], in early pregnancy can lead to neural tube defects ranging in severity from lethal anencephaly to spina bifida [1]. In adults, FAD can cause megaloblastic anemia, characterized by hypersegmented neutrophils due to impaired DNA synthesis [6].
Folate also plays a crucial role in metabolic dysfunction. The cardiovascular-kidney metabolic syndrome, previously known as the metabolic syndrome, along with its constituent features, is a newly defined concept that highlights the interconnectedness of metabolic risk factors with cardiovascular disease, chronic kidney disease, and metabolic dysfunction-associated steatotic liver disease (MASLD) [7]. MASLD, defined as steatotic liver disease associated with metabolic dysfunction, is a common metabolic disorder influenced by genetics and lifestyle habits [8-11]. It is increasingly recognized as a systemic disorder that plays a pivotal role in the development of various MASLD-related adverse hepatic and extrahepatic outcomes [12, 13]. Mechanistically, steatogenesis involves the accumulation of predominantly macrovesicular lipid vacuoles in the cytoplasm of hepatocytes. This ectopic accumulation of fats results from imbalanced dynamics between increased de novo lipogenesis and decreased beta-oxidation, as well as diminished export of triglycerides from the hepatocyte into the bloodstream [14]. Disruptions in one-carbon metabolism (OCM) have been linked to the development of metabolic dysfunction, oxidative stress, inflammation, and MASLD progression in mouse models [15]. OCM encompasses a network of metabolic pathways that provide substrates for cellular methylation reactions and are essential for synthesizing DNA, polyamines, amino acids, creatine, phospholipids, mitochondrial functionality, cellular energy homeostasis, and the assembly and secretion of very low-density lipoproteins (VLDL) from the liver [16, 17]. In line with these contemporary views, methyl-group donors such as S-adenosylmethionine were tested for clinical use in Italy since the early 1970s [18].
Beyond lifestyle changes, limited treatment options are available for MASLD, with only one drug, resmetirom, approved by the Food and Drug Administration (FDA) for the treatment of the non-cirrhotic steatohepatitic variant of MASLD, known as metabolic dysfunction-associated steatohepatitis (MASH) [19]. Interestingly, an interim analysis of a phase 3 trial has recently shown that once-weekly 2.4 mg semaglutide improved liver histological results in patients with MASH and moderate or advanced liver fibrosis [20]. Semaglutide is a synthetic glucagon-like peptide-1 receptor agonist (GLP-1 RA) that regulates glucose metabolism and gastrointestinal motility. It can be administered weekly subcutaneously or daily orally and induces a substantial reduction of visceral adiposity and body weight, decreases blood pressure, insulin resistance, and improves lipid profiles [21]. Consistent with these metabolic improvements, semaglutide carries cardiovascular benefits for patients with established atherosclerotic cardiovascular disease, lowers the risk of renal outcomes and cardiovascular-related mortality, and improves the quality of life [21]. However, the biochemical impact of semaglutide on MASLD/MASH pathogenesis remains to be fully ascertained, and a recent study by Werge et al contributes to characterizing this important aspect [15].
The study by Werge
Werge et al [15] have recently provided the most comprehensive picture to date of how OCM is disrupted across the spectrum of MASLD and how these alterations can be pharmacologically reversed by semaglutide. Leveraging targeted plasma metabolomics and bulk transcriptomics from 100 biopsy-proven MASLD patients and 50 matched healthy controls, the authors demonstrate that virtually every arm of the OCM network (i.e., methionine cycle, folate cycle, choline oxidation, transsulfuration, cysteine oxidation, and glutathione synthesis) is perturbed in human disease, with a biochemical signature of systemic oxidative stress reflected by an elevated glutamate/(serine + glycine) (GSG) index (Fig. 1a). These clinical data are mirrored by and mechanistically explained in a diet-induced obesity and steatohepatitis (DIO-MASH) mouse model. In the DIO-MASH mouse, untargeted metabolomics of liver and plasma, together with RNA-seq, reproduce the same pathway-wide suppression of OCM enzymes and loss of methyl- and thiol-donor metabolites, indicating that the murine model faithfully recapitulates the human pathophysiology (Fig. 1b). Administration of semaglutide for 12 weeks reduces body weight, hepatic inflammation and fibrosis in DIO-MASH mice. Critically, this treatment also normalizes or “over-corrects” many of the disturbed OCM metabolites (notably methionine, S-adenosyl-methionine, serine, glycine, betaine, cystathionine and cysteine) and restores transcriptional activity of key enzymes such as methionine adenosyltransferase 1α (MAT1A), glycine N-methyltransferase (GNMT), cystathionine-β-synthetase (CBS), and cystathionine-γ-lyase (CTH), while down-tuning excessive glutathione peroxidase expression. This suggests that part of semaglutide’s hepatoprotective efficacy may arise from re-establishing redox and methylation balance rather than from weight loss alone (Fig. 1c).
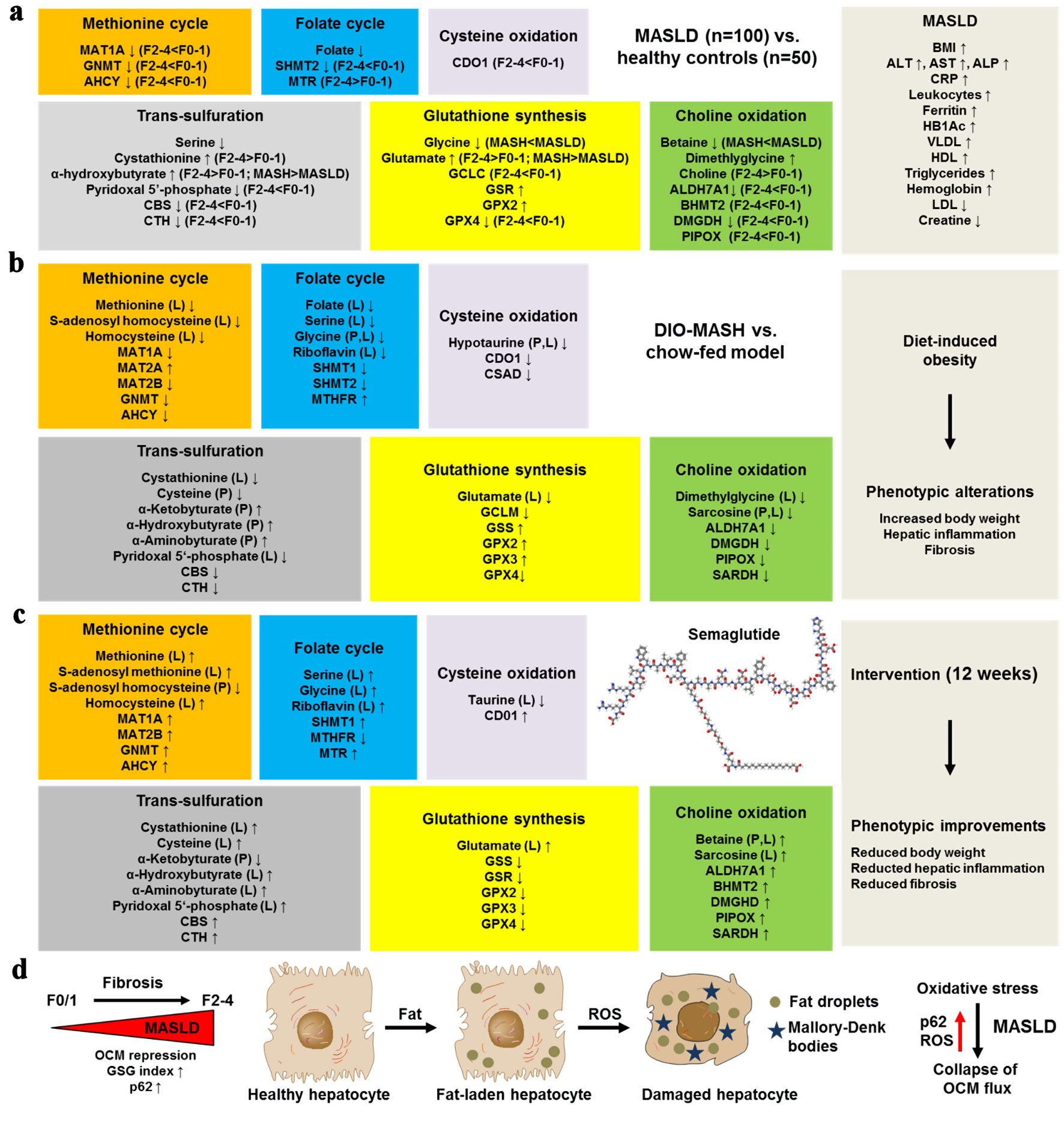 Click for large image | Figure 1. Semaglutide rebalances one-carbon metabolism (OCM) along the progression of MASLD. (a) Metabolic alterations occur in the methionine cycle, folate cycle, cysteine oxidation, choline oxidation, transsulfuration, glutathione synthesis, and choline oxidation pathways in human MASLD. This was observed in 100 biopsy-proven MASLD patients versus 50 healthy controls. (b) Similar metabolite and transcriptional changes occur in the mouse DIO-MASH model as in human MASLD. (d) In the mouse DIO-MASH model, semaglutide intervention for 12 weeks led to the normalization or “over-correction” of key metabolites. (d) MASLD and ongoing fibrosis are further associated with increased reactive oxygen species (ROS) production and increased expression of the sequestosome protein p62 in fat-laden, ballooned hepatocytes, which drive the collapse of OCM flux. AHCY: adenosylhomocysteinase; ALDH7A1: aldehyde dehydrogenase 7 family member A1; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BHMT2: betaine-homocysteine S-methyltransferase 2; BMI: body mass index; CBS: cystathionine beta synthase; CDO1: cysteine dioxygenase; CRP: C-reactive protein; CSAD: cysteine sulfinic acid decarboxylase; CTH: Cystathionine γ-lyase; DIO: diet-induced obesity; DMGDH: dimethylglycine dehydrogenase; GCLC: glutamate-cysteine ligase C; GCLM: glutamate-cysteine ligase M; GNMT: glycine N-methyltransferase; GPX2: glutathione peroxidase 2; GPX4: glutathione peroxidase 4; GSS: glutathione synthetase; GSR: glutathione-disulfide reductase; Hb1Ac: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; MAT1A/2A/2B: methionine adenosyltransferase 1A/2A/2B; MTHFR: methylenetetrahydrofolate reductase; MTR: methionine synthase; PIPOX: pipecolic acid and sarcosine oxidase; SARDH: sarcosine dehydrogenase; SHMT1/2: serine hydroxymethyltransferase 1/2; VLDL: very-low-density lipoprotein. |
Several granular observations stand out as novel findings. First, the authors pinpoint pyridoxal-5′-phosphate (active vitamin B6) deficiency as an early and progressive feature of MASLD, linking low co-factor availability to inefficient flux through transsulfuration and accumulation of the oxidative stress surrogate α-hydroxybutyrate. This association had been suggested but never substantiated in a large, histology-anchored human cohort. Second, while prior studies focused almost exclusively on homocysteine, the present work shows that homocysteine itself is not consistently elevated. Instead, its downstream metabolite cystathionine and the derived α-keto- and α-hydroxy-butyrate are markedly increased. This refines the notion that MASLD is characterized by accelerated but incomplete homocysteine disposal rather than simple hyperhomocysteinemia. Third, by stratifying patients according to fibrosis stage, the authors demonstrate a stepwise repression of GNMT, mitochondrial serine hydroxymethyltransferase (SHMT2), betaine-homocysteine methyltransferase (BHMT), CBS, CTH, and cysteine dioxygenase type 1 (CDO1) as fibrosis advances from F0/1 to F2-4. This implicates collapsing methyl-transfer and sulfide-producing capacity as potential drivers of fibrogenesis and providing a transcriptomic rationale for why OCM intermediates could serve as noninvasive fibrosis biomarkers. Fourth, the tight negative correlation between p62-positive Mallory-Denk bodies (a histological read-out of oxidative stress indicative of ballooned hepatocytes) and expression of MAT1A, GNMT and CBS suggests an intriguing feedback loop in which oxidative burden results in the collapse of OCM flux (Fig. 1d). This further compromises methyl-donor generation, thereby exacerbating epigenetic instability. This insight was previously limited to in vitro observations. Finally, the semaglutide arm furnishes the first evidence that a GLP-1 RA can reverse OCM derailment independently of choline supplementation or direct antioxidants, opening a new mechanistic window on incretin-based therapies in MASLD.
Compared to earlier metabolomic surveys that were either small, lacked histological confirmation, or only examined plasma or liver individually, this study integrates multi-omics data from two species (mice and men) and two compartments (plasma and liver). This approach overcomes common confounders such as diet, renal clearance, and sample matrix that often hinder translation across platforms. Previous animal studies had suggested that MAT1A downregulation or CBS deletion were singular events that alone can effectively drive steatosis and liver damage [22, 23]. Werge et al [15] demonstrated that this is a coordinated, pathway-level repression that can be sensitive to pharmacological intervention. This suggests that therapeutic targeting should focus on the entire OCM circuitry rather than individual enzymes. Additionally, the study reveals a nuanced role of oxidative stress. Instead of being just a by-product, redox imbalance actively influences OCM gene expression. Semaglutide appears to correct both aspects simultaneously, setting it apart from antioxidants that do not impact methyl flux.
In summary, this paper advances the field in three key areas: 1) It redefines MASLD as a disorder of integrated OCM linked to oxidative and metabolic stress; 2) It suggests that OCM-derived plasma markers such as the GSG index, cystathionine, α-hydroxybutyrate, and betaine could serve as potential biomarkers for disease progression and treatment monitoring; 3) It identifies semaglutide as the first clinically available agent capable of reshaping hepatic OCM, providing a plausible biochemical mechanism for its anti-steatotic and anti-fibrotic effects.
Conclusions
The study by Werge et al [15] has potential clinical implications that are both diagnostic and therapeutic. Additionally, the study also provides pathogenic insight and stimulates further clinical trials.
In terms of noninvasive diagnosis and disease stratification, elevated glutamate levels along with an increased GSG index, reflecting an adaptive response to increased oxidative stress, could potentially be tested in clinical practice to support clinical judgment on whether to perform a liver biopsy and upgrade a subset of individuals to more intensive treatment schedules.
From a therapeutic perspective, the study by Sanyal et al [20] offers stronger evidence supporting the use of semaglutide in MASH. However, FDA approval is necessary before semaglutide can receive an additional indication for MASH. By documenting strong oxidative stress that parallels the severity of fibrosis, the study by Werge et al [15] relaunches research on the use of antioxidants in MASH, as proposed by previous studies [24-26].
The study by Werge et al [15] also holds pathogenic significance, suggesting the existence of a liver alpha-cell axis accounting for deranged amino acid metabolism, which may have secondary consequences on gluco-lipidic homeostasis.
Collectively, these novel insights combined with previous evidence call for clinical trials that incorporate OCM endpoints to distinguish between weight-loss-dependent and direct metabolic effects of GLP-1 RAs. They also support combination strategies that pair semaglutide with methyl-donor supplementation in advanced fibrosis.
In summary, the study by Werge et al [15] provides a stimulating view of the potential role of OCM in the development and progression of MASLD in humans and how semaglutide may impact this deranged OCM homeostasis. However, additional studies are needed to determine whether the observed biochemical changes are causally related to fibrosis progression in MASH or represent its epiphenomenon.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare
Author Contributions
Both authors contributed equally to the conception, first drafting, editing, and revision of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
DIO-MASH: diet-induced obesity and steatohepatitis; FAD: Folic acid deficiency; GLP-1 RA: glucagon-like peptide-1 receptor agonist; GSG: glutamate/(serine + glycine); MASLD: metabolic dysfunction-associated steatotic liver disease; MASH: metabolic dysfunction-associated steatohepatitis; OCM: one-carbon metabolism
| References | ▴Top |
- Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25(1):27-42.
doi pubmed - Bedoui Y, Guillot X, Selambarom J, Guiraud P, Giry C, Jaffar-Bandjee MC, Ralandison S, et al. Methotrexate an old drug with new tricks. Int J Mol Sci. 2019;20(20):5023.
doi pubmed - Kozminski P, Halik PK, Chesori R, Gniazdowska E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int J Mol Sci. 2020;21(10):3483.
doi pubmed - Gleckman R, Blagg N, Joubert DW. Trimethoprim: mechanisms of action, antimicrobial activity, bacterial resistance, pharmacokinetics, adverse reactions, and therapeutic indications. Pharmacotherapy. 1981;1(1):14-20.
doi pubmed - Baddam S, Khan KM, Jialal I. Folic acid deficiency. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Killeen RB, Adil A. Macrocytic anemia. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, Coresh J, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606-1635.
doi pubmed - European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81(3):492-542.
doi pubmed - Zhang H, Zhou XD, Shapiro MD, Lip GYH, Tilg H, Valenti L, Somers VK, et al. Global burden of metabolic diseases, 1990-2021. Metabolism. 2024;160:155999.
doi pubmed - Weiskirchen R, Lonardo A. PNPLA3 as a driver of steatotic liver disease: navigating from pathobiology to the clinics via epidemiology. J Transl Genet Genom. 2024;8:355-377.
doi - Lonardo A, Singal AK, Osna N, Kharbanda KK. Effect of cofactors on NAFLD/NASH and MAFLD. A paradigm illustrating the pathomechanics of organ dysfunction. Metab Target Organ Damage. 2022;2(3):12.
doi pubmed - Goodheart RH, Ayonrinde OT. Connecting the dots: hepatic steatosis as a central player in the choreography of the liver-cardiovascular-kidney-metabolic syndrome. Heart Lung Circ. 2025.
doi pubmed - Targher G, Valenti L, Byrne CD. Metabolic dysfunction-associated steatotic liver disease. N Engl J Med. 2025;393(7):683-698.
doi pubmed - Ress C, Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J Gastroenterol. 2016;22(4):1664-1673.
doi pubmed - Werge MP, McCann A, Rashu EB, Lam SM, Hetland LE, Thing M, Nabilou P, et al. Alterations in one-carbon metabolism in metabolic dysfunction associated steatotic liver disease may be modified by semaglutide. Ann Hepatol. 2025.
doi pubmed - Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci. 2019;7:263-287.
doi pubmed - Suzuki A, Henao R, Reed MC, Nijhout F, Tripathi M, Kumar Singh B, Yen PM, et al. Lower hepatic CBS and PEMT expression in advanced NAFLD: inferencing strategies to lower homocysteine with a mathematical model. Metab Target Organ Damage. 2024;4:21.
doi - Durigato S. [S-adenosylmethionine in the therapy of liver insufficiency during a course of pulmonary tuberculosis]. Minerva Med. 1972;63(91):5024-5029.
pubmed - Lonardo A. Resmetirom: finally, the light at the end of the NASH Tunnel? Livers. 2024;4:138-141.
doi - Sanyal AJ, Newsome PN, Kliers I, Ostergaard LH, Long MT, Kjaer MS, Cali AMG, et al. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med. 2025;392(21):2089-2099.
doi pubmed - Weiskirchen R, Lonardo A. How 'miracle' weight-loss semaglutide promises to change medicine but can we afford the expense? Br J Pharmacol. 2025;182(8):1651-1670.
doi pubmed - Alarcon-Vila C, Insausti-Urkia N, Torres S, Segales-Rovira P, Conde de la Rosa L, Nunez S, Fucho R, et al. Dietary and genetic disruption of hepatic methionine metabolism induce acid sphingomyelinase to promote steatohepatitis. Redox Biol. 2023;59:102596.
doi pubmed - Lambooy S, Heida A, Joschko C, Nakladal D, van Buiten A, Kloosterhuis N, Huijkman N, et al. Selective hepatic Cbs knockout aggravates liver damage, endothelial dysfunction and ROS stress in mice fed a western diet. Int J Mol Sci. 2023;24(8):7019.
doi pubmed - Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50(6):1818-1826.
doi pubmed - Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, Tio F, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42(8):1481-1488.
doi pubmed - Bril F. Will a “multivitamin” a day keep the “MASLD doctor” away? Metab Target Organ Damage. 2025;5:44.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.