| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://cmmr.elmerpub.com |
Original Article
Volume 3, Number 1, August 2025, pages 13-24
Bovine H5N1 Hemagglutinin Could Bind Both Alpha-2,3-Linked Sialic Acid and Alpha-2,6-Linked Sialic Acid Expressed in Bovine and Large Feline Tissues, Suggesting a Zoonotic Threat
Takuma Hayashia, c, Kenji Sanob, Tetsuya Degawab, Mako Okadaa, Ikuo Konishia
aCancer Medicine, National Hospital Organization Kyoto Medical Center, Kyoto-City, Kyoto 612-8555, Japan
bDepartment of Pathology, Shinshu University Hospital, Matsumoto, Nagano 390-0877, Japan
cCorresponding Author: Takuma Hayashi, Cancer Medicine, National Hospital Organization Kyoto Medical Center, Fukakusa, Fushimi-ku, Kyoto-City, Kyoto 612-8555, Japan
Manuscript submitted May 10, 2025, accepted July 11, 2025, published online July 28, 2025
Short title: α2,3SA in Artiodactyla and Large Carnivores
doi: https://doi.org/10.14740/cmmr104
| Abstract | ▴Top |
Background: Highly pathogenic avian influenza virus (HPAI/H5N1) has caused widespread infections in dairy cattle and poultry in the United States. Sporadic cases of human HPAI/H5N1 infections have also been confirmed. To prevent human HPAI/H5N1 infections and establish treatments for infected individuals, researchers at medical institutions in the United States are analyzing the biological characteristics of HPAI/H5N1 strains confirmed in the country from March to October 2024. In particular, health care staff at such institutions are investigating human infection with the HPAI/H5N1 virus. Confirmed cases mainly involved adults who have come into contact with infected animals. HPAI/H5N1 infection causes mild illness, such as conjunctivitis, and is short-lived. Most infected patients improve or are cured with early antiviral treatment. However, on January 7, 2025, the Louisiana State Health Department reported the death of a person believed to have been infected with HPAI/H5N1 through contact with captive or wild birds. Evidence of limited human-to-human transmission of influenza A (H5N1) (via close physical contact, for example within a household) has been suggested in previous outbreaks. To date, sustained human-to-human transmission of avian influenza A (H5N1) has never been observed globally.
Methods: Our research team compared the infectivity of the HPAI/H5N1 virus to humans and dairy cows through the molecular pathological analysis of mammary gland tissues obtained from humans and dairy cows and in silico examinations.
Results: The results revealed that more receptors for HPAI/H5N1 viruses were expressed in mammary gland tissues obtained from dairy cows than human mammary gland tissues. Furthermore, in silico analysis revealed that the HPAI/H5N1 virus obtained from infected dairy cows can infect humans. In other words, the backbone molecule of the HPAI/H5N1 virus may mutate within the infected host, resulting in infectivity to humans. Furthermore, molecular pathological analysis of respiratory tissue obtained from lions revealed the expression of both human (α2,6-linked sialic acid (α2,6SA)) and avian (α2,3-linked sialic acid (α2,3SA)) influenza virus receptors in lion respiratory tissue, as well as the expression of angiotensin-converting enzyme 2, a receptor for severe acute respiratory syndrome coronavirus 2.
Conclusions: H5N1 that infects dairy cows mutates within the host and becomes infectious to humans. Therefore, it is important for people to consider and implement measures to prevent infection of dairy cows with H5N1. Therefore, One Health guidelines including zoonotic disease should be emphasized to prevent HPAIV/H5N1 infection from becoming a pandemic.
Keywords: HPAIV H5N1; Influenza virus; Mammary grand
| Introduction | ▴Top |
The highly pathogenic avian influenza (HPAI) virus (H5N1) has spread in dairy cows and pigs in the United States (USA) [1]. In September 2024, the spread of HPAI/H5N1 infections among dairy cows in the USA was reported, and the scale of the outbreak was much more serious than expected [2]. Cases of HPAI/H5N1 infection of human or livestock infections have been increasing in the country, with HPAI/H5N1 being detected in live cattle and dairy products [3]. Experts believe the virus may be evolving in conjunction with seasonal influenza virus epidemics, so monitoring the emergence of H5N1 variants and reassortants with human influenza viruses through global surveillance is essential [4]. Since March 2024, 14 cases of HPAI/H5N1 infection have been reported [5]. HPAI/H5N1 has been spreading for the past few years, causing the deaths of millions of wild birds and farmed poultry, as well as tens of thousands of marine and terrestrial mammals [6]. The development of a potentially troubling situation within the USA is currently creating increasing public concern. For example, dairy cows have shown a high HPAI/H5N1 viral load, causing recurring problems for dairy farmers with poultry. Furthermore, the continued spread of HPAI/H5N1 among dairy cows and pigs, as well as its frequent detection in raw milk, could indicate a pandemic [7, 8]. Most worryingly, in humans, HPAI/H5N1 infections have increased, and the risk of a potential pandemic has been increasing since September 2024. However, compared with the situation during the coronavirus disease 2019 (COVID-19) pandemic in 2020, we are now better prepared to prevent an emerging infectious disease, should HPAI/H5N1 infection become a viral pandemic.
In March 2024, the HPAI/H5N1 virus was detected for the first time in dairy cows in Texas, while the HPAI/H5N1 infection continues to spread among dairy cows across the country [5]. HPAI/H5N1 infection in wild bird populations has a very high mortality rate and has caused the extinction of poultry and seabirds over the past few years [9, 10]. The HPAI/H5N1 virus also causes fatal infections in many mammals that come into contact with infected birds. The Animal and Plant Health Inspection Service (APHIS) under the US Department of Agriculture (USDA) reported 675 HPAI/H5N1 infections in dairy cows across 15 states [11]. This number of infected dairy herds are only those cases known to APHIS officials. The actual number of cases may be higher. The USDA has since required HPAI/H5N1 testing for dairy cows before moving them between states [12]. Each time a human is infected, the virus gains a fresh opportunity to evolve into a form capable of infecting humans [13].
The genetic rearrangements occurring in pigs generate new influenza virus variants, so the new H5N1 variant may emerge from H5N1 variants and reassortants with human influenza viruses in dairy cows. Therefore, to understand the receptor biology of avian influenza, medical professionals and virologists must understand the virological and biological characteristics of HPAI/H5N1 and its mutant variants. Our research team compared the infectivity of HPAIV/H5N1 clade 2.3.4.4b strains in humans and dairy cows by molecular pathological analysis of mammary gland tissues collected from humans and dairy cows. In these experiments, to clarify the mechanism of virus development, we investigated the binding affinity of H5N1 mutant hemagglutinin (HA) to its receptor using molecular pathology on mammary tissues of dairy cows and humans, along with in silico analysis. In addition, to clarify the expression of receptors for human influenza viruses and HPAI/H5N1 in the respiratory organs (epithelium of the bronchioles and alveolar epithelium) of lions, which are large carnivores, we performed molecular pathological techniques using the respiratory organs of lions.
| Materials and Methods | ▴Top |
Detection of SAα2,3Gal (α2,3-linked sialic acid (α2,3SA)) and SAα2,6Gal (α2,6-linked sialic acid (α2,6SA)) in respiratory tissues, mammary gland tissues, and conjunctival tissues obtained from human, dairy cow, and lion as big feline animal
For detection of sialyloligosaccharides reactive with SAα2,3Gal- or SAα2,6Gal-specific lectins, the tissue sections were incubated with 250 µL of fluorescein isothiocyanate (FITC)-labeled Sambucus nigra (SMA) lectin (green), (Catalogue no. FL-1301-2, Vector Laboratories, Burlingame, CA, USA), biotinylated Maackia amurensis I (MAA/MAL-I) lectin (Catalogue no. B-1315-2, Vector Laboratories), or biotinylated Maackia amurensis II (MAH/MAL-II) lectin (Catalogue no. B-1365-1, Vector Laboratories) overnight at 4 °C. The sections were incubated with Alexa Fluor 594-conjugated streptavidin (red), (Catalogue no. 98036S, Molecular Probes, Inc., Eugene, OR, USA) for 2 h at room temperature. Immunostaining samples were visualized under a confocal microscope (Leica TCS SP8, Wetzlar, Germany) according to the manufacturer’s procedure. The expression levels of host receptor SAα2,3Gal or SAα2,6Gal indicated in visualized files were quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan). The quantified expression levels of host receptor SAα2,3Gal- or SAα2,6Gal were then plotted on a graph.
The expression levels of host receptor SAα2,3Gal or SAα2,6Gal were quantified by the analysis algorithm of the color program. Therefore, the expression levels of host receptor SAα2,3Gal or SAα2,6Gal are numbers expressed by a measurement system according to the manufacturer’s procedure (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan).
The analysis algorithm of the color program consists of three main steps: 1). Binarization of positive cells: Binarize positive cells using red, green, blue (RGB) or hue, lightness, saturation (HLS) numerical settings (color extraction). 2) Calculation of average brightness: Calculate the average brightness for each binarized area. For example, if one cell has 100 pixels, calculate the average brightness of 100 pixels. 3) Creation of frequency distribution table: Create a frequency distribution table based on the average brightness. Color-code the cells based on the calculated values. The range of which areas to separate the colors can be selected arbitrarily.
After receiving informed consents from patients, human respiratory tissues (including bronchial, bronchiolar, and alveolar tissues) and mammary grand tissues were obtained from normal sites of patient tissues that had been surgically removed at NHO Kyoto Medical Center.
Tissue of mammary gland
We obtained mammary gland tissues from dairy cows as by-product after meat processing, purchased from a cattle livestock farmer in Obihiro, Hokkaido, Japan.
Tissue of upper respiratory tract and lung organs from deceased lion
We purchased upper respiratory tract and lung organs from deceased lion, collected by veterinarians from Miyazaki Safari Park in Kyushu, Japan. We ordered respiratory organs from lion and mammary tissue from dairy cows from Funakoshi Co., Ltd., (Bunkyoku, Tokyo, Japan) and BuziCom Japan Co., Ltd. (Toshimaku, Tokyo, Japan), who had personnel from each organization collect respiratory tissue from deceased lion in Miyazaki Safari Park in Miyazaki Prefecture, Japan (Miyazaki-city, Miyazaki, Japan).
Related details can be found here (Supplementary Material 1, cmmr.elmerpub.com).
Solid-phase binding assay
Microtitre plates (Nunc) were incubated with fourfold serial dilutions (2.5, 0.625, 0.156, 0.039, 0.01, 0.002 and 0.001 µg/mL) of the sodium salts of sialylglycopolymers (Yamasa Corporation Co., Ltd., Choshi, Chiba, Japan) - Neu5Acα2,3Galβ1,4GlcNAcβ1-poly-Glu (α2,3SA) and Neu5Acα2,6Ga lβ1,4GlcNAcβ1-poly-Glu (α2,6SA) - in phosphate-buffered saline (PBS) at 4 °C overnight. The next day, glycopolymer solutions were removed and nonspecific binding was blocked by the addition of PBS containing 4% bovine serum albumin (BSA) at room temperature for 1 h. Plates were washed with cold PBS and then incubated with solutions containing recombinant HA proteins obtained from influenza virus variants.
To perform the solid-phase binding assay, we purchased recombinant HA proteins from various viruses, including human Isumi-H1N1 influenza virus, H5N1, Indonesia H5N1, and bovine H5N1 from the following manufacturers and distributors. We purchased HA protein obtained from Isumi-H1N1 virus from Sino Biological Inc. (BDA, Beijing 100176, China). We purchased recombinant influenza HA1 (H5N1) protein (active; ab219879) from Abcam Inc. (Waltham, MA 02453, USA). We purchased HA protein from Indonesia H1N1 (A/Indonesia/5/2005) from Sino Biological Inc. (BDA, Beijing 100176, China). We purchased HA protein obtained from bovine H5N1 from Acrobiosystems Inc. (Newark, DE 19711, USA).
Regarding the sequence of segment 4 HA gene obtained from human H1N1 virus (A/Isumi/UT-KK001-01/2018), H1N1 virus’s complete coding sequences (CDS) is described on the website of the National Center for Biotechnology Information (NCBI) (Bethesda, MD, USA). Regarding the sequence of segment 4 HA gene obtained from human H5N1 virus (A/Hong Kong/483/1997(H5N1)), H5N1 virus’s complete CDS is described on the website of NCBI (Bethesda, MD, USA). Regarding the sequence of segment 4 HA gene obtained from human Indonesia H5N1 virus (A/Indonesia/5/2005 (H5N1)), human Indonesia H5N1 virus’s complete CDS is described on the website of NCBI (Bethesda, MD, USA). Regarding the sequence of segment 4 HA gene obtained from bovine H5N1 (influenza A virus gene; A/bovine/New Mexico/A240920343-93/2024(H5N1)), bovine H5N1 virus’s complete CDS is described on the website of NCBI (Bethesda, MD, USA).
Details were provided here (Supplementary Material 2, cmmr.elmerpub.com).
Phylogenetic analysis and annotation
Reference genomes and amino acids of HA from H1N1 virus, H5N1, Indonesian H5N1 (called as Indonesian H5), bovine H5N1 (called as bovine H5) were obtained from the NCBI affiliated with National Library of Medicine (Bethesda, MD, USA).
Analyses of the three-dimensional structure of the binding site between SAα2,3Gal (α2,3SA) and SAα2,6Gal (α2,6SA) and amino acids of HA from H1N1 virus, H5N1, Indonesian H5N1 (referred to as Indonesian H5), and bovine H5N1 (referred to as bovine H5)
Spanner is a structural homology modeling pipeline that threads a query amino-acid sequence onto a template protein structure. Spanner is unique in that it handles gaps by spanning the region of interest using fragments of known structures. To create a model, we must provide a template structure, as well as an alignment of the query sequence you wish to model onto the template sequence. Spanner will replace mismatched residues, and fill any gaps caused by insertions or deletions.
We obtained the primary amino acid sequences required to construct the three-dimensional structure of the HA region of each virus from publicly available virus sequences. To construct the three-dimensional structure of the HA region of each virus, we used the publicly available virus sequences: human H1N1 virus (A/Isumi/UT-KK001-01/2018 H1N1), human H5N1 (A/Hong Kong/483/1997(H5N1)), human Indonesia H5N1 virus 192R (A/Indonesia/5/2005 (H5N1) mut), and bovine H5N1 (A/bovine/New Mexico/A240920343-93/2024(H5N1)) on the website of NCBI (Bethesda, MD, USA).
The loop region of bovine H5N1 HA binds to the host receptor α2,3SA. Therefore, we constructed the three-dimensional structure of the loop region of bovine H5N1 HA. Next, we construct the three-dimensional structure of LSTa, an analog of α2,3SA. The three-dimensional structure of the loop region of bovine H5N1 HA is docked with the three-dimensional structure of LSTa to create the complex structure. The three-dimensional structure of this complex is called basic three-dimensional structure (complex A) (Supplementary Material 3, cmmr.elmerpub.com).
We construct the three-dimensional structure of the loop region of bovine H5N1 HA. Next, we construct the three-dimensional structure of LSTc, an analog of α2,6SA. The three-dimensional structure of the loop region of bovine H5N1 HA and the three-dimensional structure of LSTc are combined to create the complex structure. The three-dimensional structure of this complex is designated as the target three-dimensional structure (complex B) (Supplementary Material 3, cmmr.elmerpub.com).
The corresponding three-dimensional structure (complex B) (Supplementary Material 3, cmmr.elmerpub.com) is superimposed on the basic three-dimensional structure (complex A) (Supplementary Material 3, cmmr.elmerpub.com). On the other hand, if the basic three-dimensional structure (complex A) (Supplementary Material 3, cmmr.elmerpub.com) and the target three-dimensional structure (complex B) (Supplementary Material 3, cmmr.elmerpub.com) do not match, the loop region of the HA of bovine H5N1 is considered not to bind to the host receptor α2,6SA. The results of this study suggest that bovine H5N1 binds to the host receptor α2,6SA for human influenza viruses. In the Supplementary data, the information is explained clearly using diagrams and charts (Supplementary Materials 4, 5, cmmr.elmerpub.com).
Statistical analyses
Differences in the expressions of avian influenza virus receptor and human influenza virus receptor between human tissues, dairy cow tissues, and lion tissues depicted in each figure were assessed using Mann Whitney U test for continuous variables, or Fisher’s exact test for categorical variables. P < 0.005 was considered significant. Statistical analyses were performed using R, version 4.0.2 (Microsoft, Redmond, WA, USA), and GraphPad Prism v.9.0.(GraphPad Software, Boston, MA, USA).
Ethical considerations
Institutional Review Board (IRB) approval and consent to participate
This research on human cancer genome information derived from results of cancer genome gene panels was conducted at Kyoto University, its affiliated hospitals, and the National Hospital Organization Kyoto Medical Center in accordance with institutional guidelines (IRB approval no. 50-201504, NHOKMC-2023-2, and H31-cancer-2). All patients were briefed on the clinical study and agreed to take part in the present study by providing informed consent for participation. Our clinical research complied with the Helsinki Statement (ethics committee name: IRB of the National Hospital Organization Headquarters (approval code: H31-cancer-2; approval date: November 9, 2019, and June 17, 2013); IRB of Kyoto University (approval code: R34005; approval date: August 1, 2022)).
The authors attended research ethics education through the Education for Research Ethics and Integrity (APRIN e-learning program (eAPRIN)) agency. The completion numbers for the authors are AP0000151756, AP0000151757, AP0000151769, and AP000351128.
ARRIVE checklist documentation
We never use live animals; the protocol of our research does not involve any live animals.
The ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) are a checklist of recommendations for the full and transparent reporting of research involving animals - maximizing the quality and reliability of published research, and enabling others to better scrutinize, evaluate and reproduce it. The guidelines ensure that studies are reported in detail enough to add to the knowledge base.
| Results | ▴Top |
Expression of the α2,3SA and α2,6SA receptors in respiratory, mammary gland, and conjunctival tissues obtained from humans and dairy cows
The expression of the HPAI/H5N1 virus receptor α2,3SA (red) and human influenza virus receptor α2,6SA (green) in human respiratory (trachea, bronchioles, and alveoli), mammary gland, and conjunctival tissues are shown in Figure 1a. The α2,6SA receptor was expressed in all tissues but particularly prominently in the bronchioles and alveoli. Furthermore, α2,6SA was weakly expressed in the human mammary gland and conjunctival tissues. Human influenza viruses enter the body and bind to the mucosal epithelial cells of the bronchioles and alveoli to establish infection. Human bronchioles and alveoli exhibited marked expression of α2,3SA (Fig. 1a, Supplementary Material 6, cmmr.elmerpub.com). Human mammary and conjunctival tissues showed weak expression of the α2,3SA receptor (Fig. 1a, Supplementary Material 6, cmmr.elmerpub.com). Similarly, HPAI/H5N1 invades the body and binds to the mucosal epithelial cells of the bronchioles and alveoli to establish infection.
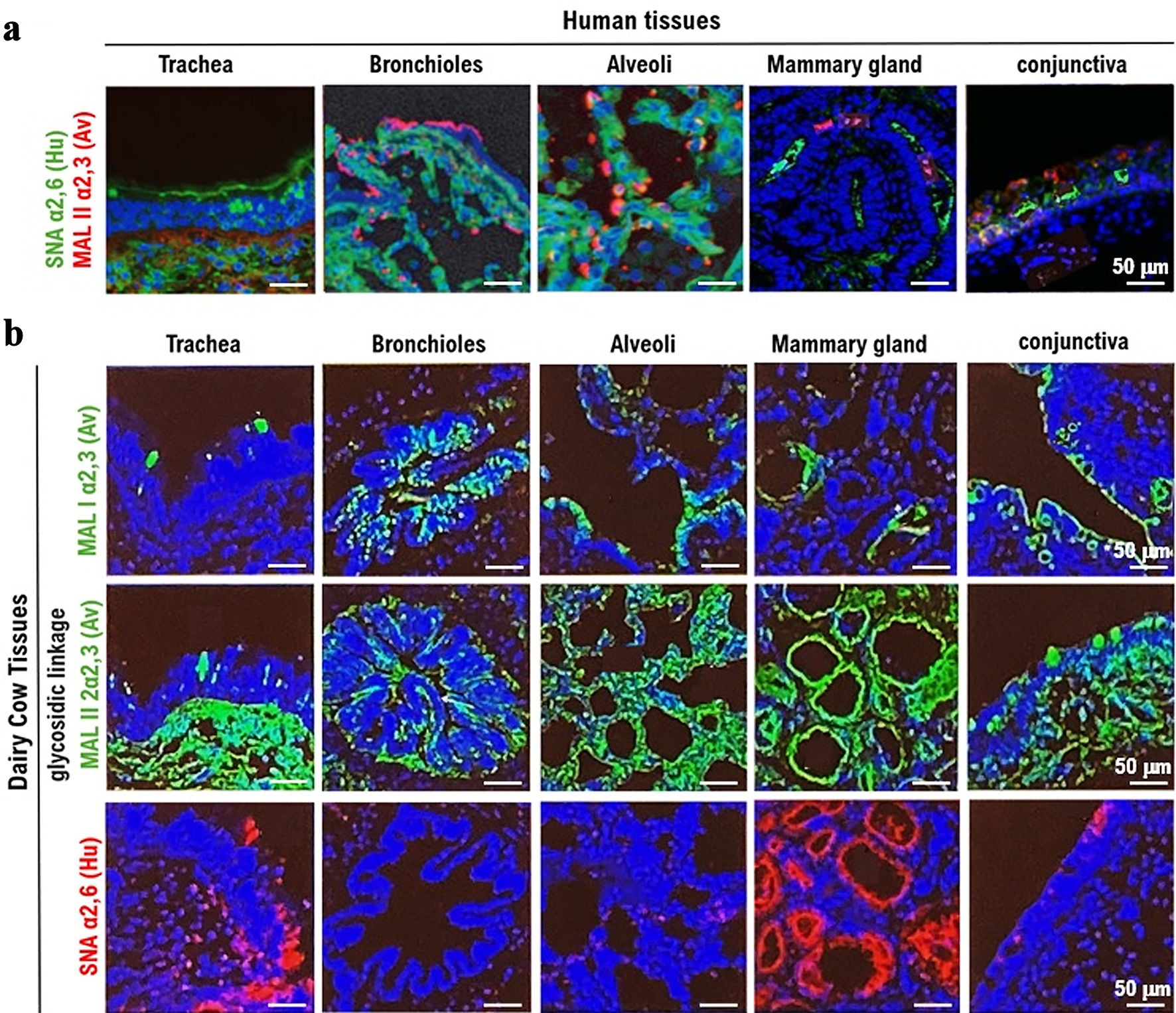 Click for large image | Figure 1. Expression of the α2,3SA receptor for HPAI/H5N1 and the α2,6SA receptor for human influenza virus in respiratory, mammary gland, and conjunctival tissues obtained from humans and dairy cows. (a) The expression of the HPAI/H5N1 virus receptor α2,3SA (red) and human influenza virus receptor α2,6SA (green) in human respiratory (trachea, bronchioles, and alveoli), mammary gland, and conjunctival tissues. α2,6SA (green) is expressed in all tissues, particularly in the bronchioles and alveoli. However, α2,6SA was weakly expressed in human mammary gland and conjunctival tissues. Green: reaction with Sambucus nigra lectin, indicating the presence of sialic acid linked to galactose by an α2,6SA-linkage (SA_2,6Gal). Red: reaction with Maackia amurensis lectin, indicating the presence of SA_2,3Gal. Cells were counterstained with DAPI. All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. The experiments were repeatedly performed with mammary tissues from five adult women. (b) The expression of α2,3SA (green) and α2,6SA (red) in respiratory (trachea, bronchioles, and alveoli), mammary gland, and conjunctival tissues obtained from dairy cows. α2,6SA (red) is strongly expressed in the mucosal epithelial tissue of the trachea and mammary gland tissue cells. The expression of α2,6SA was not detected in the bronchioles, alveoli, or conjunctiva. MAL I lectin (red) or MAL II lectin (red) is an antagonist of α2,3SA, while SMA is an antagonist of α2,6SA. All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. The experiments were repeatedly performed with mammary tissues from five dairy cows. HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; DAPI: 4',6-diamidino-2-phenylindole. |
The tissues (trachea, bronchioles, and alveoli), mammary gland, and conjunctival tissues obtained from dairy cows are shown in Figure 1b. To investigate the expression of α2,6SA, respiratory tissues were then washed with PBS and observed under a confocal laser microscope (LEICA SP8 FALCON, Wetzlar, Germany). The expression of α2,6SA was observed in the respiratory tissues, specifically the bronchioles and alveoli. To examine the expression of α2,3SA, respiratory tissues (trachea, bronchioles, and alveoli) from lions were incubated with MAL I lectin (red) or MAL II lectin (red) for 12 h at 4 °C. α2,6SA was strongly expressed in mucosal epithelial tissue of the trachea and mammary gland tissue cells. However, α2,6SA was not detected in the bronchioles, alveoli, or conjunctiva (Fig. 1b, Supplementary Material 7, cmmr.elmerpub.com). Bovine bronchioles predominantly contain avian receptors, while lung and conjunctiva tissues primarily contain avian receptors with a minor presence of human receptors. Notably, the mammary gland contains both avian and human receptors (Fig. 1b, Supplementary Material 7, cmmr.elmerpub.com). Therefore, human influenza viruses invade the body from the outside and bind to the mucosal epithelial cells of the bronchioles and alveoli to establish infection. α2,3SA was highly expressed in all bovine tissues compared with those in human tissues. Overall, compared to human mammary tissue, cow mammary tissue appears to have a higher expression of host receptors for avian influenza viruses.
Expression of the angiotensin-converting enzyme 2 (ACE2), α2,3SA, and α2,6SA receptors in respiratory tissues obtained from lions
To date, infections of HPAI/H5N1 in domestic and wild cats have been detected [14]. Early in the COVID-19 pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported to be transmitted to stray and domestic cats. Subsequently, SARS-CoV-2 has been reported to have been transmitted to big cats, such as lions and tigers, from infected zookeepers. HPAI/H5N1 is also thought to infect big feline animals such as lions and tigers. Therefore, we investigated the expression of α2,3SA, the HPAI/H5N1 receptor, and α2,6SA, the human influenza virus receptor, and ACE2, the SARS-CoV-2 receptor, in lion respiratory tissues.
To investigate the expression of the α2,6receptor for human influenza virus, respiratory tissues (bronchioles and alveoli) from lions were incubated with SMA lectin (red) for 12 h at 4 °C. The tissues were then washed with PBS and observed under a confocal laser microscope. α2,6(red) was expressed in the bronchioles and alveoli (Fig. 2, Supplementary Material 7, cmmr.elmerpub.com). To examine the expression of α2,3 receptor for HPAI/H5N1, we incubated respiratory tissues (bronchioles, alveoli) from lions with MAL I lectin (red) or MAL II lectin (red) for 12 h at 4 °C. The tissues were then washed with PBS and observed under a confocal laser microscope. α2,3 (red) was also expressed in the bronchioles and alveoli. Therefore, α2,3 and α2,6 were expressed in the facial membrane epithelial cells of respiratory tissues (bronchioles, alveoli) obtained from lions (Fig. 2, Supplementary Materials 7, 8, cmmr.elmerpub.com). Subsequently, HA from either human influenza virus or HPAI/H5N1 was applied to the respective treated tissues and incubated at 4 °C for 12 h. After incubation, we added anti-HA monoclonal antibody (green) to each treated tissue, and it was incubated at 4 °C for 12 h. After incubation, we observed the cells under a confocal laser microscope (LEICA SP8 FALCON). α2,6 and human influenza virus HA binding complex (yellow) or α2,3 and HPAI/H5N1 HA binding complex (yellow) were observed in the mucosal cells of the bronchiole and alveoli specimens. After incubation, we observed the cells under a confocal laser microscope (LEICA SP8 FALCON) (Fig. 2, Supplementary Material 7, cmmr.elmerpub.com).
 Click for large image | Figure 2. Expression of the α2,3SA, and α2.SA6 in respiratory tissues obtained from lions. Like coronavirus disease 2019 (COVID-19), there is a possibility that lions kept in zoos could in the future become infected with HPAI infectious disease through zookeepers infected with HPAI/H5N1. Therefore, we confirmed by immunohistochemical staining the expression of α2,3SA, and α2.SA6, which are the host’s receptors for H1N1 and H5N1 in the respiratory tissues (bronchioles and alveoli) obtained from lions. Bronchioles and alveoli tissues were incubated with SMA lectin (red) for 12 h at 4 °C. The tissues were then washed with phosphate-buffered saline (PBS) and observed under a confocal laser microscope. The expression of α2,6SA (red) was observed in the bronchioles and alveoli. Respiratory tissues (bronchioles, alveoli) from lions were also incubated with MAL I lectin (red) or MAL II lectin (red) for 12 h at 4 °C. The tissues were then washed with PBS and observed under a confocal laser microscope. α2,3SA (red) was also expressed in the respiratory tissues (bronchioles and alveoli). All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. The experiments were repeatedly performed with mammary tissues from five dairy cows. hHA: HA protein derived from human influenza virus (H1N1); aHA: HA protein derived from high pathogenic avian influenza virus (H5N1); HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; HA: hemagglutinin. |
Comparison of the expression of α2,6SA and α2,3SA among human, dairy cow, and lion tissues
The expression levels of host receptors α2,3SA and α2,6SA were quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan) (Fig. 3, Supplementary Material 6, cmmr.elmerpub.com). The analysis revealed that although no statistically significant difference was observed, the expression level of α2,3SA was higher than that of α2,6SA in all human or dairy cow tissues (Fig. 3a, Supplementary Materials 6, 7, cmmr.elmerpub.com). Furthermore, the expression of α2,3SA was higher than that of α2,6SA in all dairy cow tissues (Fig. 3a, Supplementary Materials 6, 7, cmmr.elmerpub.com). Therefore, HPAI/H5N1 seems to be more likely to infect dairy cows than humans. The expression of α2,6SA was higher than that of α2,3SA in lion bronchiolar and alveolar tissues (Fig. 3b, Supplementary Material 7, cmmr.elmerpub.com). This may be one of the reasons for the current spread of HPAI/H5N1 infections in dairy cows across the USA.
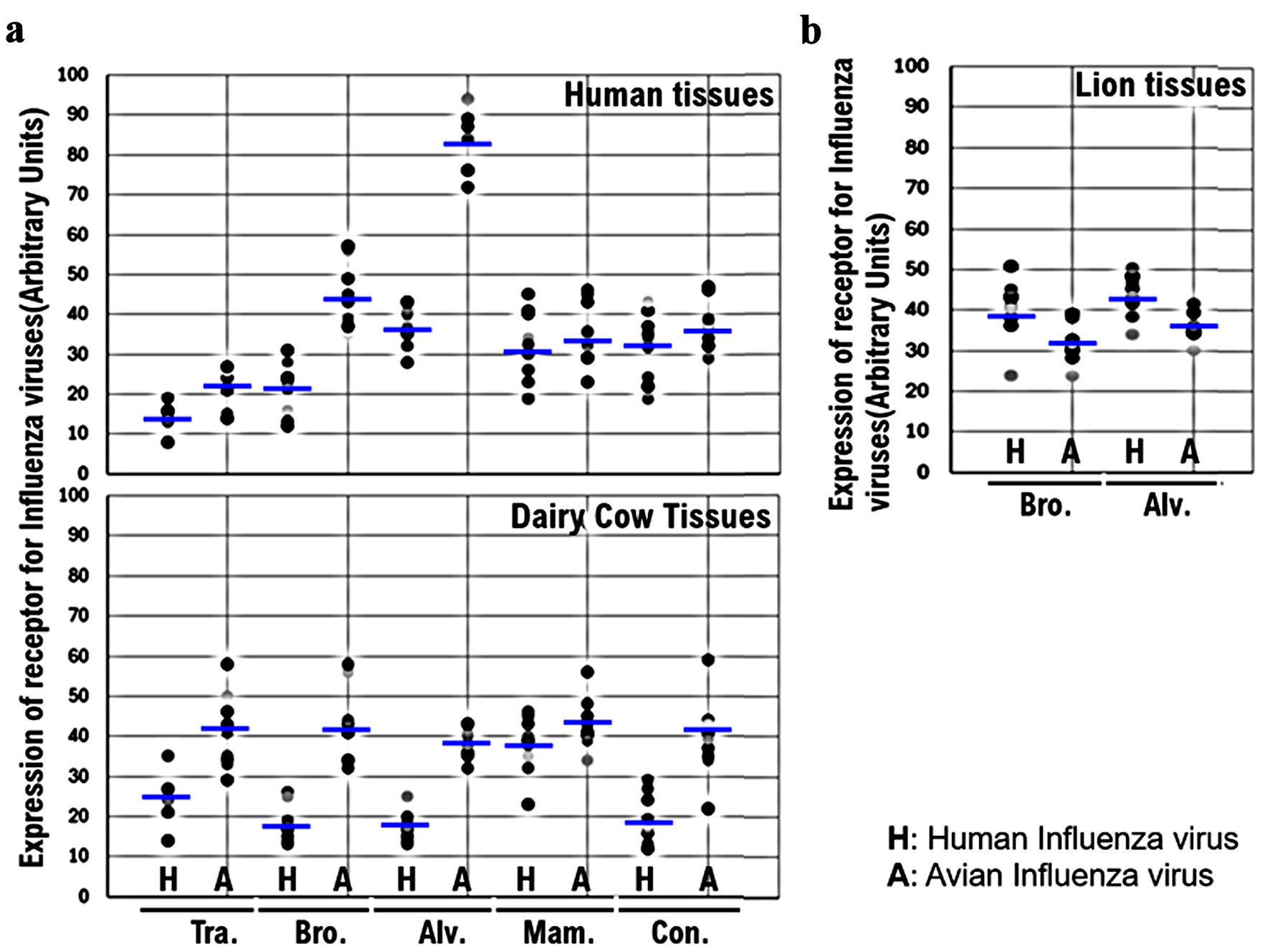 Click for large image | Figure 3. Comparison of the expression of α2,6SA and α2,3SA among human, dairy cow, and lion tissues. (a) The expression levels of host receptors α2,3SA and α2,6SA were quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan). The expression of α2,3SA was higher than that of α2,6SA in all human or dairy cow tissues. Furthermore, the expression of α2,3SA was higher than that of α2,6in all dairy cow tissues. (b) The expression of α2,6SA was higher than that of α2,3SA in the bronchiolar and alveolar tissues obtained from lions. α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid. |
Binding activity of HPAI/H5N1 variants isolated from infected dairy cows to the receptor (α2,6SA) for the human influenza virus
The HPAI/H5N1 Indonesia H5 variant, isolated from an Indonesian in 2005, is infectious to both α2,6SA and α2,3SA [15, 16]. HPAI/H5N1 has a very low possibility of infecting α2,6SA [13]. However, HPAI/H5N1 can mutate in the pig body and acquire infectivity to humans. An HPAI/H5N1 variant has been transmitted to humans from infected dairy cows. We investigated the binding affinity/recognition of HPAI/H5N1 bovine H5 variant isolated from infected dairy cows to α2,6SA or α2,3SA using an analog of either (LSTc or LSTa, respectively). The HA of bovine H5 recognized and bound to LSTc or LSTa (Fig. 4a, d). We also used HPAI/H5N1 Indonesia H5 as the control virus and examined the binding affinity/recognition of Indonesia H5 to α2,6 or α2,3 using LSTc or LSTa, respectively. The Indonesia H5 mutant, a variant of Indonesia H5, binds to α2,6SA or α2,3SA but with a weaker affinity than that of Indonesia H5. In silico analysis showed strong binding affinity/recognition between the positive control Indonesia H5 and α2,6SA or α2,3SA (Fig. 4b, e). However, Indonesia H5 mutant showed a somewhat weak binding affinity to α2,6SA or α2,3SA (Fig. 4c, f). Similar to that with Indonesia H5, the binding affinity/recognition between bovine H5 and α2,6SA or α2,3SA was observed (Fig. 4a-f). SMA lectin staining revealed that the binding affinity of bovine H5 was stronger than that of Indonesia H5 mutant, to α2,6SA or α2,3SA. In other words, HPAI/H5N1 mutated within the dairy cow’s body during infection, resulting in an HPAI/H5N1 variant that is infectious to humans.
 Click for large image | Figure 4. Binding activity of HPAI/H5N1 variants isolated from infected dairy cows to the receptor (α2,6SA) for the human influenza virus. We investigated the binding affinity/recognition of the HPAI/H5N1) variant (called as bovine H5) isolated from infected dairy cows to the α2,6SA or α2,3SA receptors using an analog of the α2,6SA receptor (LSTc) or an analog of the α2,3SA receptor (LSTa) using in silico analysis. HA of bovine H5 recognized and bound to an analog of the α2,6SA receptor (LSTc) or an analog of the α2,3SA receptor (LSTa) (a, d). In silico analysis showed strong binding affinity/recognition between the positive control Indonesia H5 (Indonesia H5 R192) and the α2,6SA or α2,3SA receptors (b, e). Indonesia H5 mutant showed somewhat weak binding (affinity) to the α2,6SA receptor or α2,3SA receptor (c, f). Indonesia H5 R192 variant is called as Indonesian H5 mut. HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; HA: hemagglutinin. |
To further test the receptor specificity of bovine H5N1, we measured the binding affinity/recognition of recombinant bovine HA to either α2,3SA or α2,6SA by performing established assays using either α2,3SA- or α2,6SA-linked sialylglycopolymers. As expected, Indonesia H5 HA showed a clear preference for α2,6SA, whereas HPAI/H5N1 HA showed a clear preference for α2,3SA (Figs. 4b, 5a). Furthermore, human Isumi-H1N1 virus HA showed non-preference for α2,3SA, whereas HPAI/H5N1 virus HA showed non-preference for α2,6SA (Fig. 5a, b). Indonesia H5 HA showed a clear preference for both α2,6SA and α2,3SA. Similarly, bovine H5 HA also showed a clear preference for both α2,3SA and α2,6SA (Fig. 5c, d). A single mutation (Q226L) within the 220-loop of H5N1 increases infectivity to humans (Supplementary Material 3, cmmr.elmerpub.com). In the Leu226 mutant, Lys156 and Asn193 make additional hydrogen bonds to the Glc-5 moiety of LSTc, which contributes to binding of LSTc to the bovine H5N1 HA (Supplementary Material 9, cmmr.elmerpub.com). Thus, HPAI/H5N1 can mutate in the dairy cow’s body and acquire the ability to infect humans.
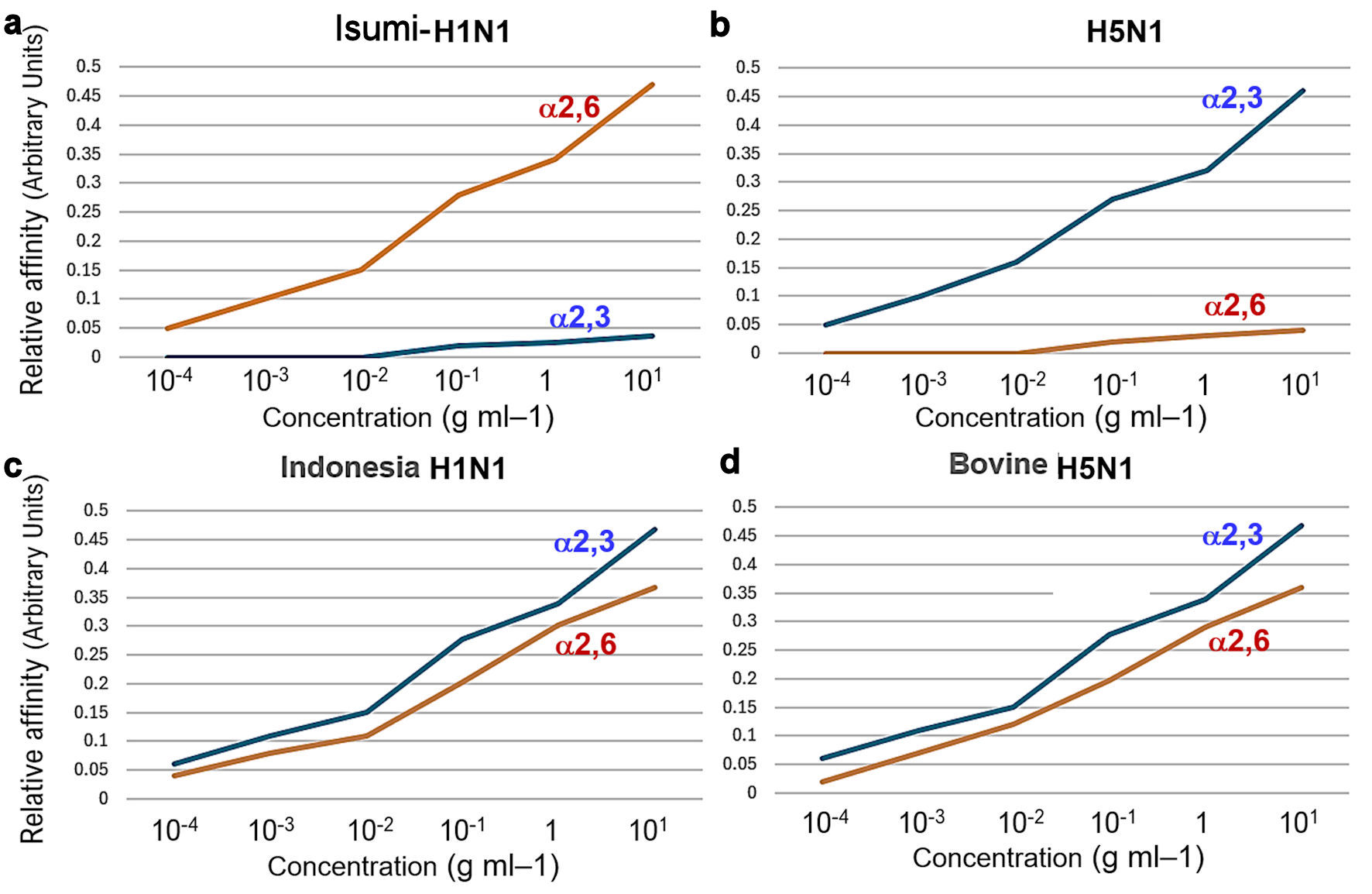 Click for large image | Figure 5. To further test the receptor specificity of bovine H5N1, we measured the binding affinity/recognition of recombinant bovine HA to either α2,3- or α2,6-linked sialic acid using established assays with either α2,3- or α2,6-linked sialylglycopolymers. Isumi-H1N1 virus HA showed a clear preference for α2,6-linked sialic acid, whereas HPAI (H5N1) virus HA also showed a clear preference for α2,3-linked sialic acid (a, b). As expected, Indonesia-human H5N1 R192 virus HA showed preference for α2,3-linked sialic acid, whereas bovine HPAI (H5N1) virus HA also showed preference for α2,6-linked sialic acid (c, d). HPAI: highly pathogenic avian influenza virus; HA: hemagglutinin. |
Comparison of α2,3SA expression between human and dairy cow mammary tissues
Paraffin-embedded human and bovine mammary tissue slices were incubated with recombinant H5N1 HA protein-His Tag for 12 h at 4 °C. The labeled anti-His Tag antibody was then added to the treated human and bovine mammary tissue slices and incubated for 12 h at 4 °C. Nuclear staining with 4',6-diamidino-2-phenylindole (DAPI) was performed. We then incubated paraffin-embedded human tissue slices with anti-human α2,6 antibody or anti-avian α2,3 antibody at 4 °C for 12 h and then stained the nuclei with DAPI. The stained tissue sections were photographed using a confocal laser microscope (LEICA SP8 FALCON), mainly after adjusting the z-axis. We then saved each image as a file and merged the image files for the DAPI, HA, and α2,3SA samples (Fig. 6a). The expression of host α2,3SA was quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation). The expression levels of host α2,3SA were then plotted on a graph (Fig. 5b). The expression of α2,3SA in bovine mammary gland tissue was approximately three times higher than that in human mammary gland tissue (Fig. 6b).
 Click for large image | Figure 6. Comparison of the expression of host receptors (α2,3SA) for HPAI/H5N1 viruses in human and dairy cow mammary tissues. (a) Paraffin-embedded human and bovine mammary tissue slices were incubated with recombinant H5N1 HA protein-His Tag for 12 h at 4 °C. Then, the labeled anti-His Tag antibody was added to the treated human and bovine mammary tissue slices and incubated for 12 h at 4 °C, and nuclear staining was performed with DAPI. We also incubated paraffin-embedded human tissue slices with anti-avian α2,3 antibody at 4 °C for 12 h and then stained the nuclei with DAPI. The stained images of each tissue section were photographed using a confocal laser microscope (LEICA SP8 FALCON). We then saved each image as a file and merged the three image files (DAPI, HA, α2,3SA). All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. (b) The expression levels of host receptor α2,3SA is quantified using an image analysis and measurement system (WinROOF2023, Mitani Corporation, Visual System, Fukui-shi, Fukui, Japan). The quantified expression levels of host receptor α2,3SA is then plotted on a graph. *P < 0.005. All photomicrographs are at × 200 magnification, and the scale bar (50 µm) applies to all panels. HPAI: highly pathogenic avian influenza virus; α2,6SA: α2,6-linked sialic acid; α2,3SA: α2,3-linked sialic acid; DAPI: 4',6-diamidino-2-phenylindole; HA: hemagglutinin. |
| Discussion | ▴Top |
According to the World Health Organization (WHO), as of mid-December 2024, 954 people have been infected with HPAIV/H5N1 worldwide, 464 (49%) of whom have died [17]. Evidence of limited human-to-human transmission of HPAI/H5N1 virus (via close physical contact, for example within a household) has been suggested in previous outbreaks. However, to date, sustained human-to-human transmission of avian HPAI/H5N1 virus has not been observed globally [18-20]. As we have found during the COVID-19 pandemic, the early production and dissemination of effective vaccines are key to preventing the spread of infection. To achieve this, medical professionals, virologists, and other experts must understand the virological and biological characteristics of HPAI/H5N1 and its mutant variants. Our research team conducted a molecular pathological analysis using human and dairy cow mammary gland tissues to compare the infectivity of the HPAI/H5N1 virus in humans and dairy cows. The results of the molecular pathological analysis revealed that the α2,3SA receptor for HPAI/H5N1 was more highly expressed in dairy cow tissues than in human tissues. In silico analysis further demonstrated that HPAI/H5N1 viruses isolated from infected dairy cows were capable of infecting humans. Similar to SARS-CoV-2, HPAI/H5N1 can infect lions. In other words, since HPAI/H5N1 and its mutant variants are both emerging and zoonotic diseases, other wild animals, livestock, and pets can also become infected by HPAI/H5N1, facilitating the spread of infection. Therefore, responding as part of One Health is a crucial preventive measure [21].
Human HPAI/H5N1 infections are extremely rare. However, when the Centers for Disease Control and Prevention (CDC) tested 115 dairy workers in Michigan and Colorado, they found that 7% of the workers had had a recent HPAIV/H5N1 infection [22]. The transmission of HPAI/H5N1 from infected dairy cows to humans has been confirmed, but no evidence indicates human-to-human transmission of HPAI/H5N1. The Indonesia H5 variant is capable of zoonotic transmission between humans and birds. Because of a mutation in the amino acid molecule in the HA, the Indonesia H5 variant is able to recognize and bind to the α2,6SA receptor for human influenza viruses and the α2,3SA receptor for HPAI/H5N1. HPAI/H5N1 can also infect non-avian species, such as pigs, and that mutations in the viral backbone molecule within the host endows the Indonesia H5 variant with infectivity to humans. Such mutations likely facilitate the spread of zoonotic viral diseases. Our in silico analysis revealed that, like the Indonesia H5 variant, the bovine variant recognizes and binds to the α2,6SA receptor for human influenza virus and α2,3SA receptor for HPAI/H5N1. In a similar mechanism, SARS-CoV-2 isolated from mink can mutate within the animal, resulting in a SARS-CoV-2 variant that is more infectious to humans. The results of infection experiments conducted by a Japanese research group have demonstrated that the highly pathogenic H5N1 avian influenza virus of bovine origin, unlike previously isolated H5N1 subtype viruses, is capable of binding to human-type receptors expressed in the upper respiratory tract of humans [23]. These findings will provide important information for developing and implementing control plans for HPAI/H5N1 and future HPAI/H5N1 variants [23].
To date, HPAI/H5N1 infections have been detected in stray cats. Therefore, like SARS-CoV-2, HPAI/H5N1 is also thought to infect big feline animals/large carnivores. We confirmed the expression of α2,3SA, a receptor for HPAI/H5N1, and α2,6SA, a receptor for human influenza virus, in respiratory tissues obtained from lions. There are concerns that infected zookeepers may be transmitting HPAI/H5N1 to big feline animals/large carnivores.
In the USA, where HPAI/H5N1 infection is spreading among cattle, the seasonal influenza virus epidemic season has already begun. Virologists are concerned that humans infected with both human influenza viruses and HPAI/H5N1 may asymptomatically produce new HPAI/H5N1 strains. The study demonstrated that a vaccine based on the HPAI/H5N1 2.3.4.4b lineage provided protection to ferrets subsequently infected with HPAI/H5N1 [23]. Because mRNA-based vaccination prevented the development of severe COVID-19 symptoms, an mRNA-based vaccine against HPAI/H5N1 may also induce the same effect. The prevalence of HPAIV/H5N1 in the USA and other countries has been confirmed not only in dairy cows but also among cats and dogs living near dairy barns. Therefore, the efficacy of an mRNA-based vaccine against HPAI/H5N1 must be established in dairy cows, cats, and dogs [24, 25].
Molecular histopathological analysis was also performed using respiratory tissue obtained from lions. The expression of α2,3SA was observed in mucosal epithelial cells of the bronchioles and alveoli. Thus, similar to SARS-CoV-2, HPAI/H5N1 infection may also affect several non-human species. In 1997, the first case of human infection with HPAI/H5N1 was confirmed. In the 2000s, people around the world feared that HPAI/H5N1 would mutate into a virus that could infect humans. More than 20 years have passed since then, but HPAI/H5N1 has still not completely mutated into human influenza. To date, only H1, H2, and H3 have undergone repeated mutations to become human influenza viruses. Some virology experts believe that HPAI/H5N1 will never become a human influenza virus. In this study, no experiments using live viruses were performed. Therefore, histopathological analysis alone can provide information on the expression of α2,3SA or α2,6SA for each virus but cannot provide accurate information on their infectivity. Future studies involving infection experiments in a biohazard level 3 infection laboratory must be conducted to understand the virological and biological properties of HPAI/H5N1 and its variants.
Conclusions
The research findings revealed higher expression of the α2,3SA receptor in bovine tissues compared to humans, while lions exhibited both α2,3SA and α2,6SA in respiratory tissues. Binding assays demonstrated that bovine H5N1 HA could bind both α2,3SA and α2,6SA, suggesting a zoonotic threat. Therefore, One Health guidelines including zoonotic disease should be emphasized to prevent HPAIV/H5N1 infection from becoming a pandemic.
| Supplementary Material | ▴Top |
Suppl 1. Detection of SAα2,3Gal (α2,3SA) and SAα2,6Gal (α2,6SA) in respiratory tissues, mammary gland tissues, conjunctival tissues obtained from human, dairy cow, and lion as big feline animal.
Suppl 2. Publicly available virus sequences: human H1N1 influenza virus, H5N1, Indonesia H1N1, and bovine H5N1.
Suppl 3. Construction of the three-dimensional complex structure of the loop region of HA docked with the three-dimensional structure of LSTa/LSTc.
Suppl 4. Phylogenetic analysis and annotation.
Suppl 5. Analyses of the three-dimensional structure of the binding site between SAα2,3Gal (α2,3SA) and SAα2,6Gal (α2,6SA) and amino acids of hemagglutinin (HA) from H1N1 virus, H5N1, Indonesian H1N1 (called as Indonesian H1), and bovine H5N1 (called as bovine H5).
Suppl 6. Expression of α2,3SA and α2,6SA in human tissues.
Suppl 7. Expression of α2,3SA and α2,6SA in the tissues obtained from dairy cows.
Suppl 8. Expression of α2,3SA and α2,6SA in the tissues obtained from lions.
Suppl 9. A single glutamine to leucine mutation at residue 226 of the virus hemagglutinin was sufficient to enact the change from avian to human specificity.
Acknowledgments
The authors want to thank Dr. Yoshiihiro Kawaoka at the Institution of Medical Science, the University of Tokyo for providing clinical research information. The authors also want to acknowledge all medical staff for clinical research at Kyoto University School of Medicine and the National Hospital Organization Kyoto Medical Center. We also appreciate the effort of FUNAKOSHI Inc. (Meguro, Tokyo, Japan) and Wakenyaku Inc. (Kyoto, Kyoto, Japan) in shipping the tissues obtained from cows and lion.
Financial Disclosure
This clinical research was performed using research funding from the following: Japan Society for Promoting Science for TH (grant no. 19K09840), START-program Japan Science and Technology Agency (JST) for TH (grant no. STSC20001), National Hospital Organization Multicenter Clinical Study for TH (grant no. 2019-Cancer in general-02), and Japan Agency for Medical Research and Development (AMED) (grant no. 22ym0126802j0001), Tokyo, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
Author Dr. Tetsuya Degawa was employed by the company DARD Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Written informed consent was obtained from all participants.
Author Contributions
TH, KS, TD, and MO were involved in the study conception and design, literature search, data collection, interpretation, and manuscript writing. TH, KS, and TD also contributed to data analysis. TH and IK, as lead physicians and medical leads for AstraZeneca, participated in study design and conduct, data evaluation, and manuscript writing and review.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kwon T, Trujillo JD, Carossino M, Machkovech HM, Cool K, Lyoo EL, Singh G, et al. Pathogenicity and transmissibility of bovine-derived HPAI H5N1 B3.13 virus in pigs. Emerg Microbes Infect. 2025;14(1):2509742.
doi pubmed - European Food Safety Authority, European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza, Alexakis L, Buczkowski H, Ducatez M, Fusaro A, et al. Avian influenza overview September-December 2024. EFSA J. 2025;23(1):e9204.
doi pubmed - Food & Beverages: Investigation of Avian Influenza A (H5N1) Virus in Dairy Cattle. U.S. Food and Drug Administration. 2025. https://www.fda.gov/food/alerts-advisories-safety-information/investigation-avian-influenza-h5n1-virus-dairy-cattle.
- Watanabe T, Kawaoka Y. Avian influenza A (H5N1) virus. The Cabinet Agency for Infectious Disease Crisis Management. https://www.caicm.go.jp/houdou/article/feature/backnumber/kako_11.html.
- Between 16 March and 14 June 2024, 42 highly pathogenic avian influenza (HPAI) A(H5) virus detections were reported in domestic (15) and wild (27) birds across 13 countries in Europe. Avian influenza overview March-June 2024, Publication series: Avian influenza overview. European Centre for Disease Prevention and Control An agency of the European Union. 2024. https://www.ecdc.europa.eu/en/publications-data/avian-influenza-overview-march-june-2024.
- Peacock TP, Moncla L, Dudas G, VanInsberghe D, Sukhova K, Lloyd-Smith JO, Worobey M, et al. The global H5N1 influenza panzootic in mammals. Nature. 2025;637(8045):304-313.
doi pubmed - Guan L, Eisfeld AJ, Pattinson D, Gu C, Biswas A, Maemura T, Trifkovic S, et al. Cow's milk containing avian influenza A(H5N1) virus - heat inactivation and infectivity in mice. N Engl J Med. 2024;391(1):87-90.
doi pubmed - Saied AA, El-Saeed BA. Infectiousness of raw (unpasteurised) milk from influenza H5N1-infected cows beyond the USA. Lancet Microbe. 2025.
doi pubmed - World Health Organization. Human infection with avian influenza A(H5) viruses. Avian Influenza Weekly Update Number 983. 2025. https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20250131.pdf.
- Pan Arica and World Health Organization. Epidemiological update avian influenza A(H5N1) in the Americas region. 2025. https://www.paho.org/sites/default/files/2025-01/2025-jan-24-phe-epiupdate-avian-influenza-eng-final.pdf.
- Schnirring L. USDA. Topics Avian Influenza (Bird Flu): USDA confirms more avian flu in US dairy cattle and poultry. News brief November 28, 2024. USDA confirms more avian flu in US dairy cattle and poultry | CIDRAP.
- United States Department of Agriculture HPAI in Livestock | Animal and Plant Health Inspection Service. 2024. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-livestock#:∼:text=This%20includes%20those%20taken%20by,against%20the%20spread%20of%20H5N1.
- Lin TH, Zhu X, Wang S, Zhang D, McBride R, Yu W, Babarinde S, et al. A single mutation in bovine influenza H5N1 hemagglutinin switches specificity to human receptors. Science. 2024;386(6726):1128-1134.
doi pubmed - Nolen RS. Feline avian influenza cases spark concerns; Cats are “exquisitely sensitive” to highly avian influenza virus, expert says. AVMA News, American Veterinary Medical Association. 2025. https://www.avma.org/news/feline-avian-influenza-cases-spark-concerns.
- Bertram S, Glowacka I, Steffen I, Kuhl A, Pohlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev Med Virol. 2010;20(5):298-310.
doi pubmed - Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440(7083):435-436.
doi pubmed - World Health Organization. Human infection with avian influenza A(H5) viruses. Avian Influenza Weekly Update Number 983. 2025. https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20250131.pdf.
- Technical Report_ June 2024 Highly Pathogenic Avian Influenza A(H5N1) Viruses _ Bird Flu _ CDC. Public Health. CDC Report. 2024. An official website of the United States government: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-06052024.html.
- Disease Outbreak News Avian Influenza A(H5N1) - Mexico. WHO report. 2025. https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON564.
- Avian influenza A(H5N1): For health professionals. Government of Canada. June 06, 2025 https://www.canada.ca/en/public-health/services/diseases/avian-influenza-h5n1/health-professionals.html.
- Ratnadass A, Deguine JP. Crop protection practices and viral zoonotic risks within a One Health framework. Sci Total Environ. 2021;774:145172.
doi pubmed - Mellis AM, Coyle J, Marshall KE, Frutos AM, Singleton J, Drehoff C, Merced-Morales A, et al. Serologic evidence of recent infection with highly pathogenic avian influenza A(H5) virus among dairy workers - Michigan and Colorado, June-August 2024. MMWR Morb Mortal Wkly Rep. 2024;73(44):1004-1009.
doi pubmed - Eisfeld AJ, Biswas A, Guan L, Gu C, Maemura T, Trifkovic S, Wang T, et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature. 2024;633(8029):426-432.
doi pubmed - Chiba S, Kiso M, Yamada S, Someya K, Onodera Y, Yamaguchi A, Matsunaga S, et al. Protective effects of an mRNA vaccine candidate encoding H5HA clade 2.3.4.4b against the newly emerged dairy cattle H5N1 virus. EBioMedicine. 2024;109:105408.
doi pubmed - Park J, Fong Legaspi SL, Schwartzman LM, Gygli SM, Sheng ZM, Freeman AD, Matthews LM, et al. An inactivated multivalent influenza A virus vaccine is broadly protective in mice and ferrets. Sci Transl Med. 2022;14(653):eabo2167.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.